Formulation and evaluation of curcumin coated central venous catheters in the eradication of catheter-related blood stream infections
DOI:
https://doi.org/10.69857/joapr.v13i3.958Keywords:
Drug-coated central venous catheters (CVC), catheter-related bloodstream infections (CRBSI), Dip coating, Curcumin, Polyurethane (PU)Abstract
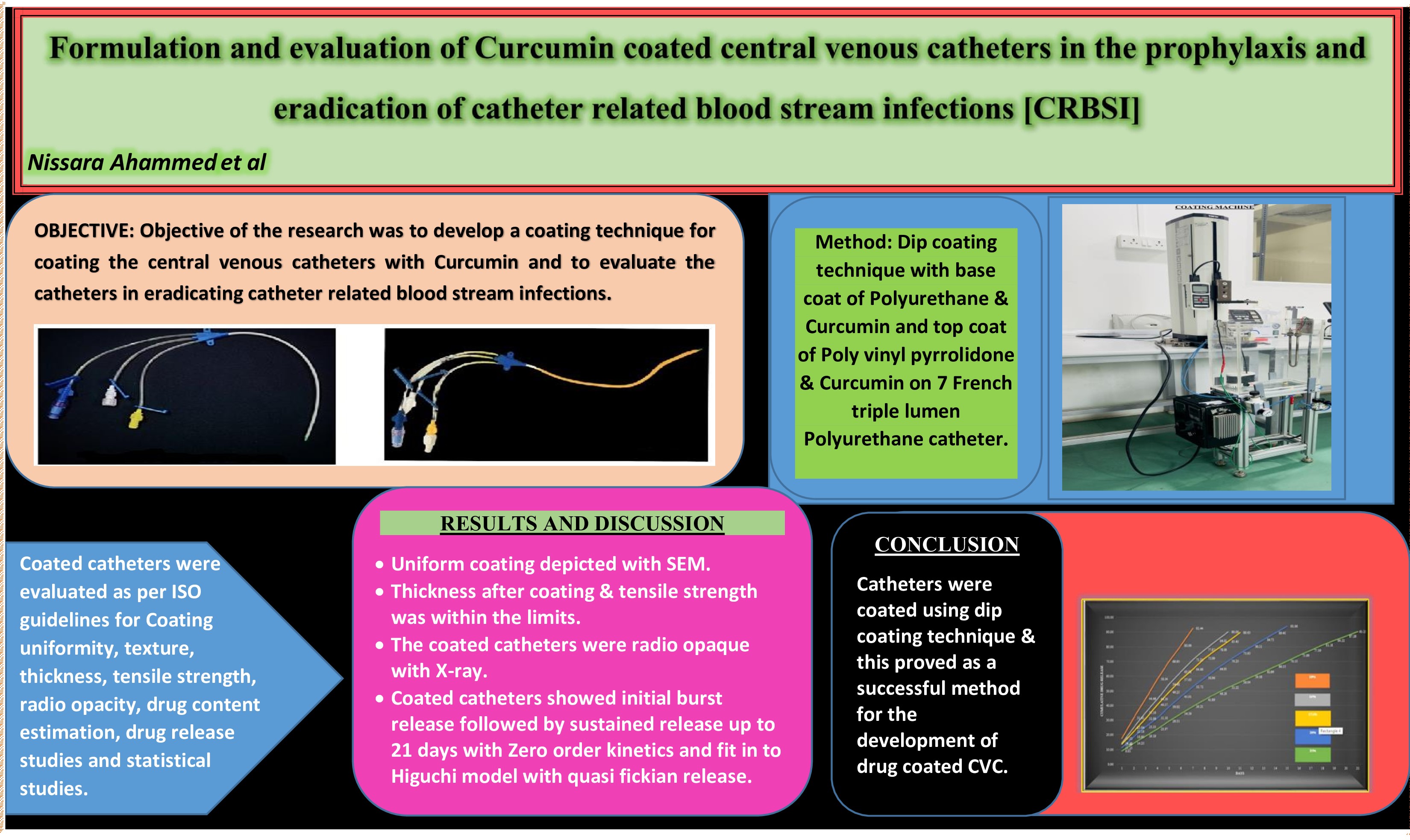
Background: Biofilm formation on catheters after implantation, leading to the development of catheter-related bloodstream infections, is a significant concern with the usage of vascular catheters, leading to the death of hospitalized patients. The research aimed to modify the catheter surface by developing a coating technique using PU, PVP, and curcumin. Methodology: A dip coating technique with a base coat of PU and a top coat of PVP was used on 7 French triple-lumen Polyurethane CVCs with the antimicrobial agent Curcumin. Based on the concentration of polyurethane used, 5 formulations were prepared and evaluated as per ISO guidelines. Results and Discussion: Dip coating was successful in coating the catheters. All the physical parameters were within the ISO limits. The process parameters that produce uniform coating were studied. The coated catheters were radiopaque. At the end of 24 hours, all formulations displayed an initial burst release due to the top coat of PVP. Formulation E demonstrated a sustained release of 94.17% of the drug over 21 days. The amount of polyurethane included in the base coat determines how long the sustained release lasts. The formulation follows zero-order kinetics and fits the Higuchi model with quasi-Fickian release, indicating that the release is diffusion-controlled. Conclusion: The dip coating technique proved to be a successful method for developing drug-coated central venous catheters. Coating with curcumin reduces bacterial colonization, growth on the catheter surface, and biofilm formation, thereby preventing CRBSI, which in turn enhances patient outcomes and lowers healthcare expenses.
Downloads
References
Hunter TB, Taljanovic MS, Tsau PH, Berger WG, Standen JR. Medical Devices of the Chest. Radiographics, 24(6), 1725-46, (2004) https://doi.org/10.1148/rg.246045031
Kehagias E, Galanakis N, Tsetis D. Central venous catheters: Which, when and how. British Journal of Radiology, 96 (1151), 1-11, (2023) https://doi.org/10.1259/bjr.20220894.
Donlan RM. Biofilms and Device-Associated Infections. Emerging Infectious Diseases., 7(2), 277-81, (2001) https://doi.org/10.3201/eid0702.010226.
Fletcher S. Catheter-related bloodstream infection. Continuing Education in Anaesthesia. Critical Care & Pain., 5(2), 49-51, (2005) https://doi.org/10.1093/bjaceaccp/mki011.
Gominet M, Compain F, Beloin C, Lebeaux D. Central venous catheters and biofilms: where do we stand in 2017? APMIS., 125(4), 365-75, (2017) https://doi.org/10.1111/apm.12665.
Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci., 4(2), 162–67 (2014) https://doi.org/10.4103/2229-5151.134184
Zhong Y, Zhou L, Liu X, Deng L, Wu R, Xia Z. Incidence risk factors and attributable mortality of catheter-related bloodstream infections in the intensive care unit after suspected catheters infection: A retrospective 10-year cohort study. Infect Dis Ther., 10, 985–99, (2021) https://doi.org/10.1007/s40121-021- 00429-3.
Brun-Buisson C. New technologies and infection control practices to prevent intravascular catheter-related infections. Am J Respir Crit Care Med., 164(9), 1557–58, (2001) https://doi.org/10.1164/ajrccm.164.9.2109051.
Pitiriga V, Kanellopoulos P, Bakalis I, Kampos E, Sagris I, Saroglouet G et al. Central venous catheter-related bloodstream infection and colonization: the impact of insertion site and distribution of multi drug resistant pathogens. Antimicrob Resist Infect Control., 9(1), 189, (2020) https://doi.org/10.1186/s13756-020-00851-1.
Maria LT, Alejandro GS, Maria Jesus PG.. Central venous catheter insertion: Review of recent evidence. Best Practice & Research Clinical Anaesthesiology., 35, 135-40, (2021) https://doi.org/10.1016/j.bpa.2020.12.009.
Wassil SK, Crill CM, Phelps SJ. Antimicrobial impregnated catheters in the prevention of catheter-related bloodstream infection in hospitalized patients. J Pediatr Pharmacol Ther., 12(2), 77-90, (2007) https://doi.org/10.5863/1551-6776-12.2.77.
Kanti SPY, Csoka I, Jojart-Laczkovich O, Adalbert L. Recent advances in antimicrobial coatings and material modification strategies for preventing urinary catheter-associated complications. Biomedicines., 10(10), 2580, (2022) https://doi.org/10.3390/biomedicines10102580.
Balikci E, Yilmaz B, Tahmasebifar A, Baran ET, Kara E. Surface modification strategies for hemodialysis catheters to prevent catheter-related infections: A review. J Biomed Mater Res B Appl Biomater., 109(3), 314-27, (2021) https://doi.org/10.1002/jbm.b.34701.
Hussain Y, Alam W, Ullah H, Dacrema M, Daglia M, Khan H et al. Antimicrobial potential of Curcumin: Therapeutic potential and challenges to clinical applications. Antibiotics., 11(3), 322, (2022) https://doi.org/10.3390/antibiotics11030322.
Qu L, Li X, Zhou J, Cao K, Xie Q, Zhou P, Qian W et al. A novel dual-functional coating based on curcumin/ a PEG polymer with antibacterial and antifouling properties. Applied Surface Science., 627, (2023) https://doi.org/10.1016/j.apsusc.2023.157224.
Chin W, Zhong G, Pu Q, Yang C, Lou W, Florez P et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nature Communications., 9(917), (2018) https://doi.org/10.1038/s41467-018-03325-6.
Zheng D, Huang C, Huang H, Zhao Y, Khan M R U, Zhao H. Antibacterial mechanism of Curcumin: A Review. Chem Biodivers., 17(8), (2020) https://doi.org/10.1002/cbdv.202000171.
Burduja N, Virzi NF, Nocito G, Ginestra G, Saita M, Spitaleri F. Curcumin-laden hydrogel coating medical device for periprosthetic joint infection prevention and control. International Journal of Pharmaceutics., 672, (2025) https://doi.org/10.1016/j.ijpharm.2025.125283.
Griffin M, Castro N, Bas O, Saifzadeh S, Butler P, Hutmacher DW. The current versatility of polyurethane three-dimensional printing for biomedical applications. Tissue Engineering Part B Reviews., 26(3), 272-83, (2020) https://doi.org/10.1089/ten.teb.2019.0224.
Feldman D. Polyurethane and polyurethane nanocomposites: Recent contributions to medicine. Biointerface Research in Applied Chemistry., 11(1), 8179-8, (2021) https://doi.org/10.33263/BRIAC111.81798189.
Bernard M, Jubeli E, Michael D, Pungente, Yagoubia N. Biocompatibility of polymer-based biomaterials and medical devices regulations in vitro screening and risk-management. Biomaterials science., 8, 1-56, (2018) https://doi.org/10.1039/C8BM00518D.
Daniel EH, Scott AG, Stuart LC. Polyurethanes. Biomaterials Science (Fourth Edition), 103(07), (2020) https://doi.org/10.1016/B978-0-12-816137-1.00010-6.
Husain MSB, Gupta A, Alashwal BY, Sharma S. Synthesis of PVA/PVP based hydrogel for biomedical applications: a review. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects., 40(20), 2388-93, (2018) https://doi.org/10.1080/15567036.2018.1495786.
Bianchi M, Pegoretti A, Fredi G. An overview of poly (vinyl alcohol) and poly(vinylpyrrolidone) in pharmaceutical additive manufacturing. J Vinyl Addit Technol., 29, 223-39, (2023) https://doi.org/10.1002/vnl.21982.
Srisang S, Nasongkla N. Layer-by-layer dip coating of Foley urinary catheters by chlorhexidine-loaded micelles. Journal of Drug Delivery Science and Technology., 4, 235-42, (2019) https://doi.org/10.1016/j.jddst.2018.11.019.
Yassina MA, Elkhooly TA, Elsherbinyd SM, Reichad FM, Shokeir AA. Facile coating of urinary catheter with bio–inspired antibacterial coating. Heliyon., 5, (2019) https://doi.org/10.1016/j.heliyon.2019.e02986.
Gomes RN, Borges I, Pereira AT. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon., 139, 635-47, (2018) https://doi.org/10.1016/j.carbon.2018.06.044.
Abbasnezhad N, Zirak N, Champmartin S, Shirinbayan M, Bakir F. An Overview of In vitro Drug Release Methods for Drug-Eluting Stents. Polymers., 14(13), 2751, (2022) https://doi.org/10.3390/polym14132751.
Srisang S, Boongird A, Ungsurungsie M, Wanasawas P, Nasongkla N. Biocompatibility and stability during storage of Foley urinary catheters coated chlorhexidine loaded nanoparticles by nanocoating: in vitro and in vivo evaluation. J Biomed Mater Res., 109(4), 496-504, (2020) https://doi.org/10.1002/jbm.b.34718.
Rampino T, Gregorini M, Germinario G, Pattonieri EF, Fulvia Erasmi et al. Extracellular vesicles derived from mesenchymal stromal cells delivered during hypothermic oxygenated machine perfusion repair ischemic/reperfusion damage of kidneys from extended criteria donors. Biology., 11(3), 350, (2022) https://doi.org/10.3390/biology11030350.
Ching YC, Mudiyanselage SU, Gunathilake, Chuah CH, Ching KY, Singh R, Liou NS. Curcumin/Tween 20-incorporated cellulose nanoparticles with enhanced curcumin solubility for nano-drug delivery: characterization and in vitro evaluation. Cellulose., 26, 5467–81, (2019) https://doi.org/10.1007/s10570-019-02445-6.
Raval A, Bahadur P, Raval A. Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating. Journal of Pharmaceutical Analysis., 8(1), 45–54, (2018) https://doi.org/10.1016/j.jpha.2017.06.002.
Mohamed AI, Abd-Motagaly AME, Osama A, Ahmed A, Amin S, Ali AIM. Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry. Pharmaceutics., 9(1), 7, (2017) https://doi.org/10.3390/pharmaceutics9010007.
Epshtein NA. Compatibility of medicinal and excipient substances in the development of medicinal formulations. Pharm Chem Journal., 52(7), 648–57, (2018) https://doi.org/10.1007/s11094-018-1876-4
ICH Topic Q8 (R2) Pharmaceutical development., 8, (2009) https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf
Rajesh R, Navya V, Kumar SK. Importance of drug excipient compatibility studies by using or utilizing or employing various analytical techniques – an overview. IJPSR., 13(9), 3473-85, (2022) https://doi.org/10.13040/IJPSR.0975-8232.13(9).3473-85.
Aminu N, Chan SY, Mumuni MA, Umar NM, Tanko N, Zauro SA, Aminu A, Toh SM. Physicochemical compatibility studies of triclosan and flurbiprofen with excipients of pharmaceutical formulation using binary, ternary, and multi-combination approach. Future Journal of Pharmaceutical Sciences., 7(148), (2021) https://doi.org/10.1186/s43094-021-00302-7.
Vasudevan S , Durai RD, Chellappan DR, Narayanan VHB , Prabu PC, Solomon AP. A polymer-based anti-quorum catheter coating to challenge MDR Staphylococcus aureus: in vivo and in vitro approaches. J Antimicrob Chemother., 74(6), 1618-26, (2019) https://doi.org/10.1093/jac/dkz094.
Arafat M, Fouladian P. Development and in vitro evaluation of 5-Fluorouracil-eluting stents for the treatment of colorectal cancer and cancer related obstruction. Pharmaceutics., 13(1), 17, (2020) https://doi.org/10.3390/pharmaceutics13010017.
Rafienia M, Zarinmehr B. Coated urinary catheter by PEG/PVA/gentamicin with drug delivery capability against hospital infection. Iranian Polymer Journal., 22, 75-83, (2013) https://doi.org/10.1007/s13726-012-0105-3.
Low JL, Kao PH, Tambyah PA, Koh GLE, Ling H, Kline KA et al. Development of a polymer-based antimicrobial coating for efficacious urinary catheter protection. Biotechnology Notes., 2. 1-10, (2021) https://doi.org/10.1016/j.biotno.2020.12.001.
Roza FN, Herliansyah MK. Preparation and characterization of Curcumin-based coating material on Co-Cr alloy. International Journal of Technology., 15(1), 18-27, (2024) https://doi.org/10.14716/ijtech.v15i1.3588.
ISO 17327-1:2018(E) Non-active surgical implants — Implant coating — Part 1: General requirements. https://cdn.standards.iteh.ai/samples/59591/732c6d91220347858cfd7595df715310/ISO-17327-1-2018.pdf
ISO 10555-1:2013(E) Intravascular catheters - Sterile & single-use catheters - Part 1: General requirements. https://cdn.standards.iteh.ai/samples/54884/66aa41ad599c4b0599d6acbe5ea4577f/ISO-10555-1-2013.pdf
ASTM-F640-12: Standard Test Methods for Determining Radiopacity for Medical Use. https://store.astm.org/f0640-23.html
Padsalgikar AD. Plastics in Medical Devices for Cardiovascular Applications. A volume in Plastics Design Library Book (2017). https://books.tarbaweya.org/static/documents/uploads/pdf/Plastics-in-Medical-Devices-for-Cardiovascular-Applications-A-volume-in-Plastics-Design-Library.pdf
Archana M, Rubini D, Dharshini KP, Hari BNV, Jayasankari S, Ramyadevi D, Gonciarz W, Domanska A, Brzezinski M, Nithyanand P. Development of an anti-infective urinary catheter composed of polyvinyl alcohol/sodium alginate/methylcellulose/polyethylene glycol by using a pressure-assisted 3D-printing technique. Int J Biol Macromol., 249, (2023) https://doi.org/10.1016/j.ijbiomac.2023.126029.
Kim CR, Jang EB, Hong SH, Yoon YE, Huh BK, Kim SN et al. Indwelling urinary catheter assembled with lidocaine-loaded polymeric strand for local sustained alleviation of bladder discomfort. Bioeng Transl Med., 6(2), 1-12, (2021) https://doi.org/10.1002/btm2.10218.
Zhang S, Liyun Wan, Liang X, Vorstius J, Keatch R, Corner G, Nabi G et al. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater Sci Eng., 5(6), 2804-14, (2019) https://doi.org/10.1021/acsbiomaterials.9b00071.
Raval A, Bahadur P, Raval A. Effect of non-ionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable Polymeric coating. Journal of Pharmaceutical Analysis., 8(1), 45–54, (2018) https://doi.org/10.1016/j.jpha.2017.06.002.
Ching YC, Mudiyanselage T. Curcumin/Tween 20-incorporated cellulose nanoparticles with enhanced curcumin solubility for nano-drug delivery: characterization and in vitro evaluation. Cellulose., 26, 5467-81, (2019) https://doi.org/10.1007/s10570-019-02445-6.
Woolford S, Tran M, Yoda C, Oktem B, NguyenPho A, McDermott M, Wickramasekara S. Studying the effect of drug-to-excipient ratio on drug release profile for drug coated balloons. International Journal of Pharmaceutics., 620, (2022) https://doi.org/10.1016/j.ijpharm.2022.121749.
Dai C, Lin J, Li H, Shen Z, Wang Y, Velkov T, Shen J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants., 11(3), 459, (2022) https://doi.org/10.3390/antiox11030459.
Praditya D, Kirchhoff L, Bruning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective Properties of the Golden Spice Curcumin. Front Microbiol., 3(10), 912, (2019) https://doi.org/10.3389/fmicb.2019.00912.
Kumar M, Chandra U, Garg A, Gupta P. Investigation of Drug-Excipient Compatibility Studies Using Validated RP-HPLC Method for Azelnidipine and Telmisartan Tablets. Journal of Pharmaceutical Research International., 33(41B), 233-242, (2021) https://doi.org/10.9734/JPRI/2021/v33i41B32363.
Feng Y, Xiao K, He Y, Du B, Hong J, Yin H, Lu D et al. Tough and biodegradable polyurethane-curcumin composited hydrogel with antioxidant, antibacterial and antitumor properties. Materials Science & Engineering C., 121, (2021) https://doi.org/10.1016/j.msec.2020.111820.
Ruiz D, Uscategui YL, Diaz L, Arrieta-Perez RR, Gomez-Tejedor JA, Valero MF. Obtention and Study of Polyurethane-Based Active Packaging with Curcumin and/or Chitosan Additives for Fruits and Vegetables—Part I: Analysis of Morphological, Mechanical, Barrier, and Migration Properties. Polymers., 15(22), 4456, (2023) https://doi.org/10.3390/polym15224456.
Kravanja KA, Finsgar M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients. Materials & Design., 217, (2022). https://doi.org/10.1016/j.matdes.2022.110653.
Maeng WY, Yoon JH, Kim DJ. Effect of process conditions (withdrawal rate and coating repetition) on morphological characteristics of sol–gel TiO2 film during dip coating. Journal of Coatings Technology and Research., 17, 1171–93, (2020) https://doi.org/10.1007/s11998-020-00337-0.
Sun X, Chen P, Mujahid M, Zhou L. Effect of withdrawal speed on the microstructure, optical, and self-cleaning properties of TiO2 thin films. Journal of Sol-Gel Science and Technology., 93, 62–69, (2020) https://doi.org/10.1007/s10971-019-05171-4.
Kakaei K, Esrafili MD, Ehsani A, Chapter 8 - Graphene and Anticorrosive Properties, Interface Science and Technology., 27, 303-37, (2019) https://doi.org/10.1016/B978-0-12-814523-4.00008-3.
Won DS, Lee H, Park Y, Chae M, Kim YC, Lim B, Kang MH et al. Nanoengineered Urinary Catheters for Enhanced Antimicrobial Efficacy and Reduced Cytotoxicity. Adv. Healthcare Mater., 13, 1-15, (2024) https://doi.org/10.1002/adhm.202401700.
Bovas BC, Karunamoorthy L, Chuan FB. Effect of surface roughness and process parameters on mechanical properties of fabricated medical catheters. Materials Research Express., 6(12), (2020) https://doi.org/10.1088/2053-1591/ab6652.
Li H, Sureda A, Devkota HP, Pittala V, Barreca D, Silva AS et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv., 38, (2020) https://doi.org/10.1016/j.biotechadv.2019.01.010.
Cam ME, Yildiz S, Alenezi H, Cesur S, Ozcan GS, Erdemir O , Edirisinghe U et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: an in vitro and in vivo comparison study. Journal of Royal Society Interface., 17, (2020) https://doi.org/10.1098/rsif.2019.0712.
Ghasemiyeh P, Samani SM. Polymers Blending as Release Modulating Tool in Drug Delivery. Frontiers in Materials., 8, (2021) https://doi.org/10.3389/fmats.2021.752813.
Lowinger MB, Barrett SE, Zhang F, Williams RO. Sustained Release Drug Delivery Applications of Polyurethanes. Pharmaceutics., 10(55), (2018) https://doi.org/10.3390/pharmaceutics10020055.
Briggs F, Browne D, Asuri P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci., 23, 4118, (2022) https://doi.org/10.3390/ijms23084118.
Hariyadi DM, Hendradi E, Kurniawan TD. Alginate Microspheres Encapsulating Ciprofloxacin HCl: Characteristics, Release and Antibacterial Activity. Int J Pharma Res Health Sci., 7(4), 3020-27, (2019) https://doi.org/10.21276/ijprhs.2019.04.02
Savaser A, Tas C, Bayrak Z, Ozkan CK, Ozkan Y. Effect of different polymers and their combinations on the release of metoclopramide HCl from sustained-release hydrophilic matrix tablets. Pharmaceutical Development and Technology., 18(5), 1122–30, (2013) https://doi.org/10.3109/10837450.2012.710240.
Korsmeyer R.W, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of Solute Release from Porous Hydrophilic Polymers. International Journal of Pharmaceutics., 15, 25-35, (1983) http://dx.doi.org/10.1016/0378-5173(83)90064-9.
Stekhova Y, Kodur V, Lowe G, Baird J, Lowe K, Elhindi J et al. Role of a radiopaque agent and surveillance radiographs for peripherally inserted central catheters in newborn infants. Pediatric Radiology., 53, 2235-44, (2023) https://doi.org/10.1007/s00247-023-05705-7.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Nissara Ahammed, Revathi Sundaramoorthi, Venkatesh Dinnekere Puttegowda, Umashankar Chikkanna

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















