Development of a fast-acting nanosuspension nasal drop using a novel co-processed polymer for migraine relief
DOI:
https://doi.org/10.69857/joapr.v13i3.940Keywords:
Nanosuspension, Nasal Drop, Prochlorperazine Maleate, Migraine treatment, BioavailabilityAbstract
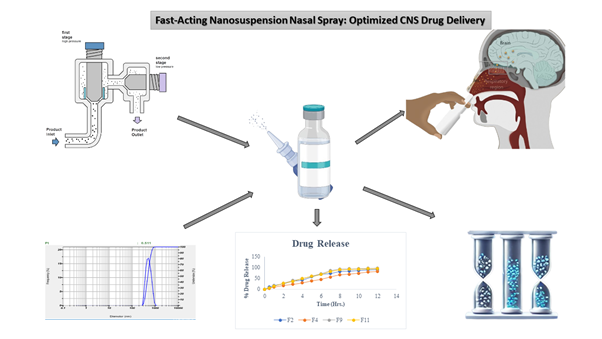
Background: Effective central nervous system (CNS) drug delivery remains challenging due to the blood-brain barrier. Nasal drug delivery offers a non-invasive alternative, ensuring rapid drug absorption and onset of action. Prochlorperazine Maleate, a drug for migraines, suffers from poor solubility, limiting its therapeutic potential. Methodology: A nanosuspension-based nasal drop was developed and optimized using high-pressure homogenization. A novel co-processed polymer enhances solubility and stability. Key formulation parameters, including particle size, zeta potential, and polymer concentration, were optimized using a central composite design. The optimized nanosuspension was characterized for its physicochemical properties, drug release, and stability. Results and Discussion: The optimized formulation (Batch F9) exhibited a particle size of 78.8 nm and a high drug release rate (93.87% in 8 hours). Stability studies confirmed no significant changes in drug content, pH, or osmolality over a three-month period. The nasal drop provided consistent dosing, with each actuation delivering a precise amount of drug content. In vitro drug release studies demonstrated a sustained release pattern, enabling prolonged migraine relief. Conclusion: The developed nanosuspension nasal drop presents a promising solution for CNS drug delivery, ensuring rapid and sustained therapeutic outcomes. This nanosuspension nasal drop achieved a 5.6-fold enhancement in solubility and demonstrated rapid onset within 10 minutes post-administration. Although promising, the study is limited to in vitro characterization; future research should explore in vivo efficacy and long-term safety.
Downloads
References
Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C. The Global Burden of Neurological Disorders: Translating Evidence into Policy. Lancet Neurol., 19(3), 255–65 (2020) https://doi.org/10.1016/S1474-4422(19)30411-9.
Patel V, Chavda V, Shah J. Nanotherapeutics in Neuropathologies: Obstacles, Challenges, and Recent Advancements in CNS Targeted Drug Delivery Systems. Curr. Neuropharmacol., 19(5), 693–710 (2021) https://doi.org/10.2174/1570159X18666200807143526.
Omidi Y, Kianinejad N, Kwon Y, Omidian H. Drug Delivery and Targeting to Brain Tumors: Considerations for Crossing the Blood-Brain Barrier. Expert Rev. Clin. Pharmacol., 14(3), 357–81 (2021) https://doi.org/10.1080/17512433.2021.1887729.
Kaur G, Goyal J, Behera PK, Devi S, Singh SK, Garg V, Mittal N. Unraveling the role of chitosan for nasal drug delivery systems: a review. Carbohydr. Polym. Technol. Appl., 5, 100316 (2023) https://doi.org/10.1016/j.carpta.2023.100316.
Patel HP, Chaudhari PS, Gandhi PA, Desai BV, Desai DT, Dedhiya PP, Vyas BA, Maulvi FA. Nose to brain delivery of tailored clozapine nanosuspension stabilized using (+)-alpha-tocopherol polyethylene glycol 1000 succinate: Optimization and in vivo pharmacokinetic studies. Int. J. Pharm., 600, 120474 (2021) https://doi.org/10.1016/j.ijpharm.2021.120474.
Parmar PK, Rao SG, Bansal AK. Co-processing of small molecule excipients with polymers to improve functionality. Expert Opin. Drug Deliv., 18(7), 907–28 (2021) https://doi.org/10.1080/17425247.2021.1873946.
Kothawade SN, Pande VV. Formulation and Evaluation of Amisulpride Loaded Intranasal Microemulsion. Indian Drugs, 60(9) (2023) https://doi.org/10.53879/id.60.09.13783.
Sharma HS, Muresanu DF, Nozari A, Lafuente JV, Tian ZR, Sahib S, Bryukhovetskiy I, Bryukhovetskiy A, Buzoianu AD, Patnaik R, Wiklund L. Pathophysiology of Blood-Brain Barrier in Brain Tumor: Novel Therapeutic Advances Using Nanomedicine. Int. Rev. Neurobiol., 151, 1–66 (2020) https://doi.org/10.1016/bs.irn.2020.03.001.
Wang X, Wu C, Liu S, Peng D. Combinatorial Therapeutic Strategies for Enhanced Delivery of Therapeutics to Brain Cancer Cells through Nanocarriers: Current Trends and Future Perspectives. Drug Deliv., 29(1), 1370–83 (2022) https://doi.org/10.1080/10717544.2022.2069881.
Proulx ST. Cerebrospinal Fluid Outflow: A Review of the Historical and Contemporary Evidence for Arachnoid Villi, Perineural Routes, and Dural Lymphatics. Cell. Mol. Life Sci., 78(6), 2429–57 (2021) https://doi.org/10.1007/s00018-020-03706-5.
Kothawade SN, Chaudhari PD. Development of Biodegradable Porous Starch Foam for Improving Oral Delivery of Eprosartan Mesylate. J. Adv. Sci. Res., 12(03), 120–26 (2021) https://doi.org/10.55218/JASR.s1202112314.
Sadekar SS, Bowen M, Cai H, Jamalian S, Rafidi H, Shatz-Binder W, Lafrance-Vanasse J, Chan P, Meilandt WJ, Oldendorp A, Sreedhara A. Translational Approaches for Brain Delivery of Biologics via Cerebrospinal Fluid. Clin. Pharmacol. Ther., 111(4), 826–34 (2022) https://doi.org/10.1002/cpt.2531.
Rasmussen MK, Mestre H, Nedergaard M. Fluid Transport in the Brain. Physiol. Rev., 102(2), 1025–51 (2022) https://doi.org/10.1152/physrev.00031.2020.
Scarpa M, Stegemann S, Hsiao WK, Pichler H, Gaisford S, Bresciani M, Paudel A, Orlu M. Orodispersible Films: Towards Drug Delivery in Special Populations. Int. J. Pharm., 523(1), 327–35 (2017) https://doi.org/10.1016/j.ijpharm.2017.03.018.
Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in Orodispersible Films for Drug Delivery. Expert Opin. Drug Deliv., 8(3), 299–316 (2011) https://doi.org/10.1517/17425247.2011.553217.
Khalid GM, Selmin F, Musazzi UM, Gennari CG, Minghetti P, Cilurzo F. Trends in the Characterization Methods of Orodispersible Films. Curr. Drug Deliv., 18(7), 935–46 (2021) https://doi.org/10.2174/1567201818999201210212557.
Witika BA, Smith VJ, Walker RB. Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions. Pharmaceutics, 12(4), 367 (2020) https://doi.org/10.3390/pharmaceutics12040367.
Zafar F, Jahan N, Ali S, Jamil S, Hussain R, Aslam S. Enhancing Pharmaceutical Potential and Oral Bioavailability of Allium cepa Nanosuspension in Male Albino Rats Using Response Surface Methodology. Asian Pac. J. Trop. Biomed., 12(1), 26–38 (2022) https://doi.org/10.4103/2221-1691.331792.
Parmar VJ, Jadeja YS, Bhandole A. A Review of the Preparation and Evaluation of Herbal Nasal Spray. J. Pharmacogn. Phytochem., 12(5), 1–4 (2023) https://doi.org/10.22271/phyto.2023.v12.i4b.14705.
Scherließ R. Nasal Formulations for Drug Administration and Characterization of Nasal Preparations in Drug Delivery. Ther. Deliv., 11(3), 183–91 (2020) https://doi.org/10.4155/tde-2019-0086.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Nikhil Shrisunder, Prashant Kumar Dhakad, Ritu Gilhotra

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















