A study on design & development of betamethasone acetate and betamethasone sodium phosphate extended-release suspension
DOI:
https://doi.org/10.69857/joapr.v13i3.922Keywords:
Betamethasone sodium phosphate (BSP), Betamethasone acetate (BA), Sterilization, Suspension, Extended-ReleaseAbstract
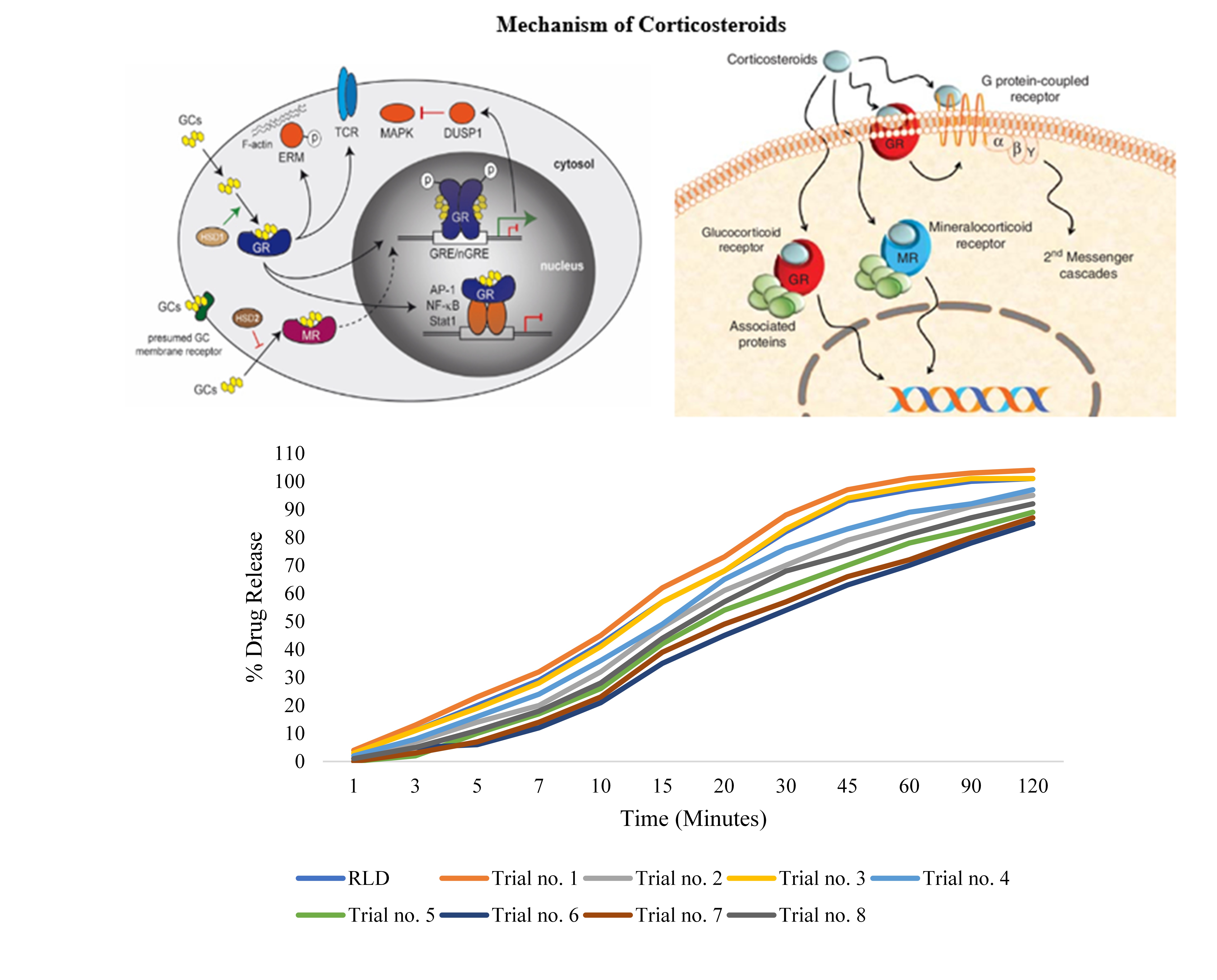
Background: The development of an injectable composition of betamethasone sodium phosphate and betamethasone acetate with an equivalent drug release profile to the marketed reference drug product, "Celestone Soluspan®," is highly challenging. To overcome this drug development problem, there is a need for a practical methodology for the preparation and evaluation of injectable compositions. Methodology: Different sterilization methods (Dry Heat Sterilization and Autoclave) and phase methods (two- or three-phase methods) are used for the preparation of the injectable composition of betamethasone sodium phosphate and betamethasone acetate. Two-phase or three phase methods and order of addition of excipients during the preparation of the formulation are the unique methodology of the present study and plays an important role in the stability of the composition. The release profile of the developed formulations is determined by using a USP type IV dissolution apparatus (STF buffer pH 7.4 as dissolution medium, 6.0 ml/min flow rate for 120 min), and stability study is also performed. Results and Discussion: As per the results of the present study Trial no. 3 shows betamethasone freebase 2.68% and total impurities 3.52% at 40°C /75% RH for 90 days and also gives similar release profile (f2 value 95%) as compared to the marketed formulation/RLD (Reference Listed Drug) i.e. Celestone Soluspan®. Conclusion: Present study concludes that injectable suspension of betamethasone sodium phosphate and betamethasone acetate using dry heat sterilisation of betamethasone acetate and three-phase method shows superior results or equivalent release profile as compared to the RLD and the key features of the present study.
Downloads
References
Reichardt SD, Amouret A, Muzzi C, Vettorazzi S, Tuckermann JP, Lühder F. Reichardt HM. The role of glucocorticoids in inflammatory diseases. Cells, 10(11), 2921 (2021) https://doi.org/10.3390/cells10112921.
Bradford RJ, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin. Therap., 39, 2216-29 (2017) http://dx.doi.org/10.1016/j.clinthera.2017.09.011.
Makkar JK, Singh PM, Jain D, Goudra B. Particulate vs non-particulate steroids for transforaminal epidural steroid injections: systematic review and meta-analysis of the current literature. Pain Physician, 19, 327-40 (2016) https://www.painphysicianjournal.com/current/pdf?article=MjkwMg%3D%3D&journal=98.
Donohue NK, Tarima SS, Durand MJ, Wu H. Comparing pain relief and functional improvement between methylprednisolone and dexamethasone lumbosacral transforaminal epidural steroid injections: a self-controlled study. Kor. J. Pain, 33(2), 192–98 (2020) https://doi.org/10.3344/kjp.2020.33.2.192.
Schneider B, Varghis N, Kennedy DJ. Ideal corticosteroid choice for epidural steroid injections: a review of safety and efficacy. Curr. Phys. Med. Rehabil. Rep., 3(2), 151-58 (2015) https://doi.org/10.1007/s40141-015-0086-1.
Ho MJ, Jeong MY, Jeong HT, Kim MS, Park HJ, Kim DY, Lee HC, Song WH, Kim CH, Lee CH, Choi YW, Choi YS, Han YT, Kang MJ. Effect of particle size on in vivo performances of long-acting injectable drug suspension. J. Contr. Release, 341, 206–14 (2022) https://doi.org/10.1016/j.jconrel.2021.12.011.
Lin X, Yang H, Su L, Yang Z, Yang X. Effect of size on the in vitro/in vivo drug release and degradation of exenatide-loaded PLGA microspheres. J. Drug Delivery Sci. and Tech., 45, 346-56 (2018) https://doi.org/10.1016/j.jddst.2018.03.024.
Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev., 8 (2013) https://doi.org/10.1002/14651858.CD006764.pub3.
Milani P Z, Ghanbarzadeh S, Basmenji S, Valizadeh H. Comparative bioequivalence study of two marketed formulations of betamethasone injectable suspensions. Drug Res. (Stuttg), 63(10), 545-49 (2013) https://doi.org/10.1055/s-0033-1348223.
Bainbridge JS. Betamethasone: friend (soluble), foe (particulate), or either?. Pain Med.,10(2), 420 (2009) https://doi.org/10.1111/j.1526-4637.2009.00574.x.
Takahashi T, Fee EL, Takahashi Y, Saito M, Yaegashi N, Usuda H, Furfaro L, Carter S, Schmidt AF, Newnham JP, Jobe AH, Kemp MW. Betamethasone phosphate reduces the efficacy of antenatal steroid therapy and is associated with lower birthweights when administered to pregnant sheep in combination with betamethasone acetate. Am. J. Obstet. Gynecol. 226(4), 564.e1-564.e14 (2022) https://doi.org/10.1016/j.ajog.2021.10.001.
Liu H, Ji M, Xiao P, Gou J, Yin T, He H, Tang X, Zhang Y. Glucocorticoids based prodrug design: Current strategies and research progress. Asian J. Pharm. Sci., 19(3), 1-38 (2024) https://doi.org/10.1016/j.ajps.2024.100922.
Águas R, Mahdi A, Shretta R, Horby P, Landray M, White L, The CoMo Consortium. Potential health and economic impacts of dexamethasone treatment for patients with COVID-19. Nat. Commun., 12(1), 1-8 (2021) https://doi.org/10.1038/s41467-021-21134-2.
Javier RV, Laura RV, Manuel GN. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. Sci., 516(1-2), 342–51 (2017) https://doi.org/10.1016/j.ijpharm.2016.11.053.
Fung AT, Tran T, Lim LL, Samarawickrama C, Arnold J, Gillies M, Catt C, Mitchell L, Symons A, Buttery R, Cottee L, Tumuluri K, Beaumont P. Local delivery of corticosteroids in clinical ophthalmology: a review. Clin. Exp. Ophthalmol., 48(3), 366–401 (2020) https://doi.org/10.1111/ceo.13702.
Meduri GU, Annane D, Confalonieri M, Chrousos GP, Rochwerg B, Busby A, Ruaro B, Meibohm B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. J. Intensive Care Med., 46(12), 2284–96 (2020) https://doi.org/10.1007/s00134-020-06289-8.
Hardy RS, Raza K, Cooper MS. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol., 16(3), 133–44 (2020) https://doi.org/10.1038/s41584-020-0371-y.
Pinson K, Gyamfi-Bannerman C. Antenatal steroids and tocolytics in pregnancy. Obstet. and Gynecol. Clin. North America, 50(1), 109-19 (2023) https://doi.org/10.1016/j.ogc.2022.10.006.
Gosar A, Folane S, Pawar S, Gharat M. Development and validation of new analytical method for the determination of particle size distribution of metformin hydrochloride using laser based particle size analyzer. J. Pharm. Res. Int., 17(5), 1-9 (2017) https://doi.org/10.9734/JPRI/2017/33911.
Prabhu AA, Vishwanath BA. Design and evaluation of injectable suspension containing anti-inflammatory glucocorticoids. J. Pharm. Res. 18(2), 9–16 (2019 https://dx.doi.org/10.18579/jopcr/v18.2.anil.
Xie F, Ji S, Cheng Z. In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example. Eur. J. Pharm. Sci., 66(23), 163-72 (2015) https://doi.org/10.1016/j.ejps.2014.10.002.
Ke X, Ping QN, Liao ZG. Interconversion kinetic studies of betamethasone acetate polymorphs in water. Drug Dev. Ind. Pharm., 32, 1019–24 (2006) https://doi.org/10.1080/03639040600734965.
Kamat M, DeLuca PP. Pharmaceutical dosage forms - parenteral medications: formulation and packaging. (Nema S, Ludwig JD Eds.) Vol. 1 (3rd ed.), CRC Press, pp.103-4 (2010) https://doi.org/10.3109/9781420086447.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Amit Bansal, Satish Sardana, Tarun Wadhwa

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















