A review on the bioactive compounds of Rhus chinensis Mill. native to the Sikkim Himalayas
DOI:
https://doi.org/10.69857/joapr.v13i3.897Keywords:
Rhus chinensis Mill., Hepatoprotective, Anti-inflammatory, Anti-oxidant, AntidiarrhealAbstract
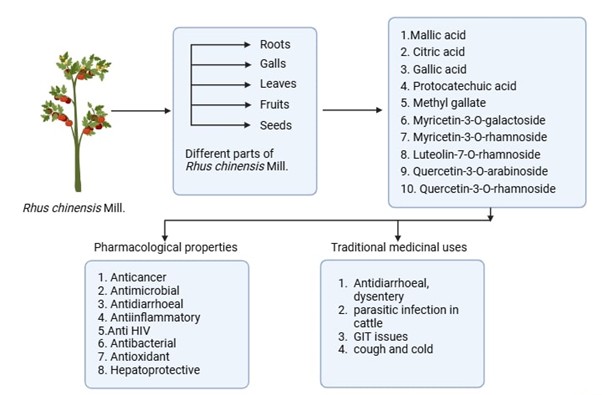
Background: The Sikkim Himalaya is home to the wild medicinal shrub Rhus chinensis Mill, which produces edible fruits. Traditionally, the fruit juice concentrate has been used to treat a variety of stomach issues. The plant is rich in phytoconstituents, including gallic acid (up to 130.4 ± 2.5 mg/g), methyl gallate, flavonoids, and tannins, which contribute to its traditional applications in managing conditions such as diarrhoea, dysentery, toothache, cough, and wounds. The total flavonoids and flavonol levels were quantified as rutin equivalents. The total phenolics were calculated as gallic acid equivalents. Through various in vitro antioxidant methods, including DPPH, Total antioxidant content, ABTS, and Hydrogen peroxide scavenging assays, the antioxidant capacity was determined. Methodology: This review combines data from numerous research studies and review articles that have elaborated on the various phytoconstituents, medicinal uses, and pharmacological properties of different Rhus species. Results and Discussion: This review provides a detailed description of multiple phytoconstituents, traditional uses, and medicinal applications of Rhus species. The quantitative findings from previous studies report the total phenolic content as 123.52±1.29 mg GAE/g. IC50 values through DPPH free radical scavenging assay and Hydrogen scavenging assay were 42.69±0.1% and 63.20±1.48% respectively. Conclusion: This review provides an in-depth description of various phytoconstituents, including gallic acid, citric acid, myricetin-3-O-rhamnoside, methyl gallate, quercetin-3-O-arabinoside, and protocatechuic acid, among others. These results provide concrete evidence to support the potential of Rhus chinensis Mill. as a source of bioactive compounds for the creation of new treatments.
Downloads
References
Anna K, Kyriakos K, Ioannis N. Co-amorphous solid dispersions for solubility and absorption improvement of drugs: Composition, preparation, characterization and formulations for oral delivery. Pharmaceutics, 10(3), 98 (2018) https://doi.org/10.3390/pharmaceutics10030098
Andre H, Johanna M, Hanlin L, Christian J, Andrea M, Bart H et.al. Challenges and strategies for solubility measurements and dissolution method development for amorphous solid dispersion formulations. AAPS J., 25(1), 11 (2022) https://doi.org/10.1208/s12248-022-00760-8
Thomas WYL, Nathan AB, Hui HW, Chow SF, Wan KY, Albert HLC. Delivery of poorly soluble compounds by amorphous solid dispersions. Curr Pharm Des., 20(3), 303-24 (2014) http://dx.doi.org/10.2174/13816128113199990396
Hou HH, Aniruddha R, Keyur MP, Joseph WL, Ariel M, Edward Y, Wei J, Karthik N. Impact of method of preparation of amorphous solid dispersions on mechanical properties: comparison of coprecipitation and spray drying. J Pharm Sci., 108(2), 870-9 (2019) https://doi.org/10.1016/j.xphs.2018.09.008
Kramarczyk D, Knapik-Kowalczuk J, Kurek M, Jamróz W, Jachowicz R, Paluch M. Hot Melt Extruded Posaconazole-Based Amorphous Solid Dispersions—The Effect of Different Types of Polymers. Pharmaceutics, 15(3), 799 (2023) https://doi.org/10.3390/pharmaceutics15030799
Xiangyu M, Robert OW. Characterization of amorphous solid dispersions: An update. J Drug Deliv Sci Technol., 50, 113-24 (2019) https://doi.org/10.1016/j.jddst.2019.01.017
Wenling F, Wenjing Z, Xinyi Z, Yan X, Liuqing D. Application of the combination of ball-milling and hot-melt extrusion in the development of an amorphous solid dispersion of a poorly water-soluble drug with high melting point. RSC Adv., 9, 22263-73 (2019) https://doi.org/10.1039/C9RA00810A
Wenjing Z, Wenling F, Xiaotong Z, Meiqi G. Sustained-release solid dispersion of high-melting-point and insoluble resveratrol prepared through hot melt extrusion to improve its solubility and bioavailability. Molecules, 26, 4982 (2021) https://doi.org/10.3390/molecules26164982
Nicole M, Bjad A, Venkata RK, Sandeep S, Priyanka T, Suresh B, Michael AR. Manufacturing strategies to develop amorphous solid dispersions: An overview. J Drug Deliv Sci Technol., 55, 101459 (2020) https://doi.org/10.1016/j.jddst.2019.101459
Jiawei H, Mengyuan T, Yang Y, Wen S, Zhimin Y, Yunran Z, Yijun Z, Xiaoqian L, Jue W. Amorphous solid dispersions: Stability mechanism, design strategy and key production of hot melt extrusion. Int J Pharm., 646 (2023) https://doi.org/10.1016/j.ijpharm.2023.123490
Muralidhar P, Dani LY, Sai KAV, Krishnamurthy B, Koteshwara KB, Srinivas M. Effervescence induced amorphous solid dispersions with improved drug solubility and dissolution. Pharm Dev Technol., 28(2), 176-89 (2023) https://doi.org/10.1080/10837450.2023.2172039
Chia MK, Wai KN, Parijat K, Kok PC, Yuancai D. Hot-melt extrusion microencapsulation of quercetin for taste-masking. J Microencapsul., 34(1), 29-37 (2017) https://doi.org/10.1080/02652048.2017.1280095
Huanyue Z, Yu W, Shuting L, Ming L. Improving chemical stability of resveratrol in hot melt extrusion based on formation of eutectic with nicotinamide. Int J Pharm., 607, 121042 (2021) https://doi.org/10.1016/j.ijpharm.2021.121042
Nabil KA, Ameeduzzafar Z, Syed SI, Khalid SA, Sultan A, Tilal E, Fadhel AA, Sultan A, Usama AF, Nabil AA, Mohammed SA. Formulation of amorphous ternary solid dispersions of dapagliflozin using PEG 6000 and Poloxamer188: Solid-state characterization, Ex-vivo study, and molecular simulation assessment. Drug Dev Ind Pharm., 46(9), 1458-67 (2020) https://doi.org/10.1080/03639045.2020.1802482
Khalid AA, Pradeep RV, Francesco T, Roberta C. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech., 12(1), 279-86 (2011) https://doi.org/10.1208/s12249-011-9584-3
Khalid AM, Mohammed AA, Shahid J, Ramadan AS, Mohammad NA, Faiyaz S. Development and evaluation of PLGA polymer-based nanoparticles of quercetin. Int J Bio Macromol., 92, 213-9 (2016) https://doi.org/10.1016/j.ijbiomac.2016.07.002
Gangqi H, Bing W, Mengli J, Shuxin D, Wenxuan Q, Yuxuan M, Zhimei M, Yuhao Q, Wenxing Z, Xinli L, Wei L. Optimization and evaluation of resveratrol amorphous solid dispersions with a novel polymeric system. Math Biosci Eng., 19(8), 8019-34 (2022) https://doi.org/10.3934/mbe.2022375
Jaywant NP, Rahul TS, Avinash BG, Kailas KM, Sharadchandra DJ, Divakar RJ, Purnima DA. Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying: Physicochemical and in silico studies. Asian J Pharm Sci., 11(3), 385-95 (2016) https://doi.org/10.1016/j.ajps.2015.08.012
Meena MK, Choudhary D, Chouhan M, Shukla P, Sinha SK. Enhancement of solubility and dissolution rate of erlotinib hydrochloride by solid dispersion technique with poloxamer 188: preparation and in-vitro evaluation. Int J Pharm Sci Res., 11(1), 387-93 (2020) https://doi.org/10.13040/ijpsr.0975-232.11(1).387-93
Siok-Yee C, Yin-Ying C, Xin-Zi C, Eryn YT, Joan Q. The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers. Asian J Pharm Sci., 10(5), 372-85 (2015) https://doi.org/10.1016/j.ajps.2015.04.003
Sakkal M, Arafat M, Yuvaraju P, Beiram R, Aburuz S. Preparation and characterization of theophylline controlled release matrix system incorporating poloxamer 407, stearyl alcohol, and hydroxypropyl methylcellulose: A novel formulation and development study. Polymers, 16(5), 643 (2024) https://doi.org/10.3390/polym16050643
Hussain T, Paranthaman S, Rizvi SMD, Moin A, Gowda DV, Subaiea GM, Ansari M, Alanazi AS. Fabrication and Characterization of Paclitaxel and Resveratrol Loaded Soluplus Polymeric Nanoparticles for Improved BBB Penetration for Glioma Management. Polymers, 13(19), 3210 (2021) https://doi.org/10.3390/polym13193210
Doreth M, Hussein MA, Priemel PA, Grohganz H, Holm R, Diego HL, Rades T, Lobmann K. Amorphization within the tablet: Using microwave irradiation to form a glass solution in situ. Int J Pharm., 519(1-2), 343-51 (2017) http://dx.doi.org/10.1016/j.ijpharm.2017.01.035
Sumit K, Brian L, Yin-Chao T. A new combination approach of CI jet and QESD to formulate pH-susceptible amorphous solid dispersions. Int J Pharm., 466, 368–74 (2014) https://doi.org/10.1016/j.ijpharm.2014.03.042
Hector P, David Q, Juan DF, Camila MM, Etelvino HBJ, Luis AG, Sandra M. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater., 2012, 145380 (2012) https://doi.org/10.1155/2012/145380
Mayur B, Zaved AK. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: preparation, characterization and pharmacokinetics and biodistribution studies in wistar rats. Int J Nanomedicine, 10(1), 3921-35 (2015) https://doi.org/10.2147/IJN.S80706
Yaning S, Fan Y, Keyu L, Qianru H, Ming M. Characterizations and bioavailability of dendrimer-like glucan nanoparticulate system containing resveratrol. J Agric Food Chem., 68(23), 6420-9 (2020) https://doi.org/10.1021/acs.jafc.0c01315
Saad MA, Wenli L, Jun-Bom P, Joseph TM, Bader BA, Soumyajit M, Nigel L, Karl K, Andreas G, Michael AR. Stability-enhanced hot-melt extruded amorphous solid dispersions via combinations of soluplus and HPMCAS-HF. AAPS PharmSciTech., 16, 824-34 (2015) https://doi.org/10.1208/s12249-014-0269-6
Zafar A, Alruwaili NK, Imam SS, Alsaidan OA, Alkholifi FK, Alharbi KS, Mostafa EM, Alanazi AS, Gilani SJ, Musa A, Alshehri S, Rawaf A, Aliquraini A. Formulation of Genistein-HP _ Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics, 13(12), 1997 (2021) https://doi.org/10.3390/pharmaceutics13121997
Xianbao S, Na F, Gang Z, Jin S, Zhonggui H, Jing L. Quercetin amorphous solid dispersions prepared by hot melt extrusion with enhanced solubility and intestinal absorption. Pharm Dev Technol., 25(4), 472-81 (2021) https://doi.org/10.1080/10837450.2019.1709502
Published
How to Cite
Issue
Section
Copyright (c) 2025 Sujan Banerjee, Bhupendra Shrestha, Surabhi Mandal

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















