A bioanalytical method development and validation for quantification of glycopyrrolate and neostigmine in rat plasma by LC-MS and its application to pharmacokinetic study
DOI:
https://doi.org/10.69857/joapr.v13i3.834Keywords:
LC-MS, Glycopyrrolate, Neostigmine, Pharmacokinetics StudiesAbstract
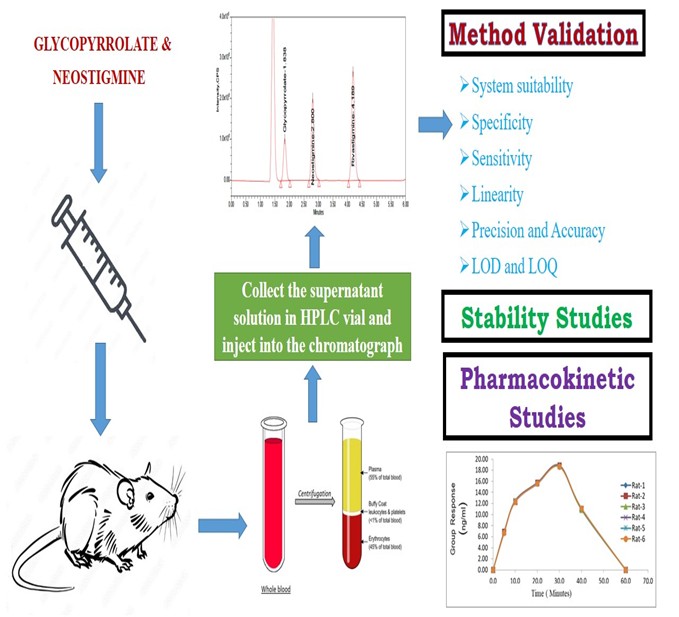
Background: After surgery, non-depolarizing neuromuscular blocking medications include neostigmine (NEO) and glycopyrrolate (GLY). Numerous traditional approaches, such as HPLC and UPLC procedures, are established for the quantification of GLY and NEO; nevertheless, they lack sensitive and specific analysis, especially in complex matrices. Using the LC/MS approach, this work develops a bioanalytical method for quantifying both drugs in rat plasma and applies it to pharmacokinetic studies. Methodology: The plasma was extracted using acetonitrile, and Rivastigmine was employed as an internal standard. An MRM method with positive ions was used for multiple reactions. A C18 column and a mobile phase - 70:30 mixture of acetonitrile and buffer was utilised at a flow rate of 1 ml/min. Plasma vortex for 10 minutes and centrifuged at 4000 rpm at 20°C. Validation and stability studies are conducted according to the ICH guidelines. The pharmacokinetic study by WinNonlin (Version 5.2) software. Results and Discussion: Rt for Glycopyrrolate and Neostigmine at 1.838 and 2.800min. GLY has a precision (%CV) of 0.45 at HQC and 3.57 at LQC. NEO had a precision (%CV) of 1.13 at HQC and 2.79 at LQC. From 2 to 40 ng/mL of GLY and 10 to 200 ng/mL of NEO, the standard curves showed a linear relationship. LOD and LOQ for both drugs were 3pg/mL and 10pg/mL. Conclusion: A simple, affordable, reliable, and sensitive approach for quantifying GLY and NEO in rat plasma using LC-MS, with Rivastigmine serving as the internal standard, was developed, validated, and successfully applied in the pharmacokinetic study of rat plasma.
Downloads
References
Polaka, S, Swetha, K. L, Dittakavi, S., Rajpoot, K, & Tekade, R. K. (2021). Bioanalytical method development and validation for establishing bioavailability and bioequivalence. Elsevier eBooks, pp. 487–516 (2021) https://doi.org/10.1016/b978-0-12-814425-1.00001-2
Arnold WA, Blum A, Branyan J, Bruton TA, Carignan CC, Cortopassi G, Datta S, DeWitt J, Doherty A-C, Halden RU, Harari H, Hartmann EM, Hrubec TC, Iyer S, Kwiatkowski CF, LaPier J, Li D, Li L, Ortiz JGM, Salamova A, Schettler T, Seguin RP, Soehl A, Sutton R, Xu L, Zheng G. Quaternary Ammonium Compounds: A Chemical Class of Emerging Concern. Environmental Science & Technology., 57(20), 7645–7890 (2023) https://doi.org/10.1021/acs.est.2c08244
Tang FPW, Leung GNW, Wan TSM. Analyses of quaternary ammonium drugs in horse urine by capillary electrophoresis - mass spectrometry. Electrophoresis., 22(11) , 2201–2209 (2001)
https://doi.org/10.1002/1522-2683(20017)22:11<2201::AID-ELPS2201>3.0.CO;2-S
Ermer J, Vogel M. Applications of hyphenated LC‐MS techniques in pharmaceutical analysis. Biomedical Chromatography., 14 (6) , 373–83 (2000)
https://doi.org/10.1002/1099-0801(200010)14:6<373::AID-BMC29>3.0.CO;2-S
Nowatzke W, Woolf E. Best Practices during Bioanalytical Method Validation for the Characterization of Assay Reagents and the Evaluation of Analyte Stability in Assay Standards, Quality Controls, and Study Samples. AAPS J, 9(13), 117–22 (2007) https://doi%3A%2010.1208/aapsj0902013.
P. Vasu Babu, D. Akila Devi. Stability Indicating Assay Method Development and Validation by RP-UPLC with PDA detector for Simultaneous Estimation of Glycopyrrolate and Neostigmine in Pharmaceutical dosage form. Research Journal of Pharmacy and Technology, 16(7), 3219-2 (2023) https://doi.org/10.52711/0974-360X.2023.00529.
Agarwal B, Jagdale S, Kadam P, Sakpal P, Nalawade S, Maske S, Dale P. Development and Validation of Stability Indicating RP-HPLC Method for the Estimation of Glycopyrrolate and Neostigmine in Bulk and Injection: Journal of Chromatographic Science, 62(3), 213–21 (2023) https://doi.org/10.1093/chromsci/bmad035
Parab M, Shirsat VA, Kodgule YM, Kodgule M. A RP-HPLC Method for the Analysis of Neostigmine Methylsulfate and Process-Related Impurities, Forced Degradation Studies, in the Injection Formulation. International Journal of Analytical Chemistry, 2021(14), 5570173, (2021) https://doi.org/10.1155/2021/5570173
Khan H. Analytical Method Development in Pharmaceutical Research: Steps involved in HPLC Method Development. Asian Journal of Pharmaceutical Research, 7(3), 203-207 (2017)
http://dx.doi.org/10.5958/2231-5691.2017.00031.4
Agarwal B, Jagdale S, Kadam P, Sakpal P, Nalawade S, Maske S, Dale P. Development and Validation of Stability Indicating RP-HPLC Method for the Estimation of Glycopyrrolate and Neostigmine in Bulk and Injection. Journal of Chromatographic Science, 62(3), 213–21 (2023) https://doi.org/10.1093/chromsci/bmad035
Tripathy SK. Pharmaceutical validation: A quality maintaining tool for pharmaceutical industry. Asian Journal of Pharmaceutical Research, 10 (4), 307–11 (2020) http://dx.doi.org/10.5958/2231-5691.2020.00052.0
Sutar SV, Yeligar VeerendraC, Patil SS. A Review: Stability Indicating Forced Degradation Studies. Research Journal of Pharmacy and Technology, 12(2), 885-90 (2019) http://dx.doi.org/10.5958/0974-360X.2019.00152.5
Dickinson L, Boffito M, Back D, Else L, von Hentig N, Davies G, Khoo S, Pozniak A, Moyle G, Aarons. Sequential Population Pharmacokinetic Modeling of Lopinavir and Ritonavir in Healthy Volunteers and Assessment of Different Dosing Strategies. Antimicrob Agents Chemotherapy, 55(60), (2011) https://doi.org/10.1128/aac.00887-10
Published
How to Cite
Issue
Section
Copyright (c) 2025 P. Vasu Babu, D. Akiladevi

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















