Phytochemical profiling and antioxidant assessment of Cassia auriculata leaves via GC-MS
DOI:
https://doi.org/10.69857/joapr.v13i3.1247Keywords:
Phytochemicals, Total Phenolics Content, Total Flavonoid Content, Antioxidant, DPPH Assay, Gas chromatography-mass spectrometryAbstract
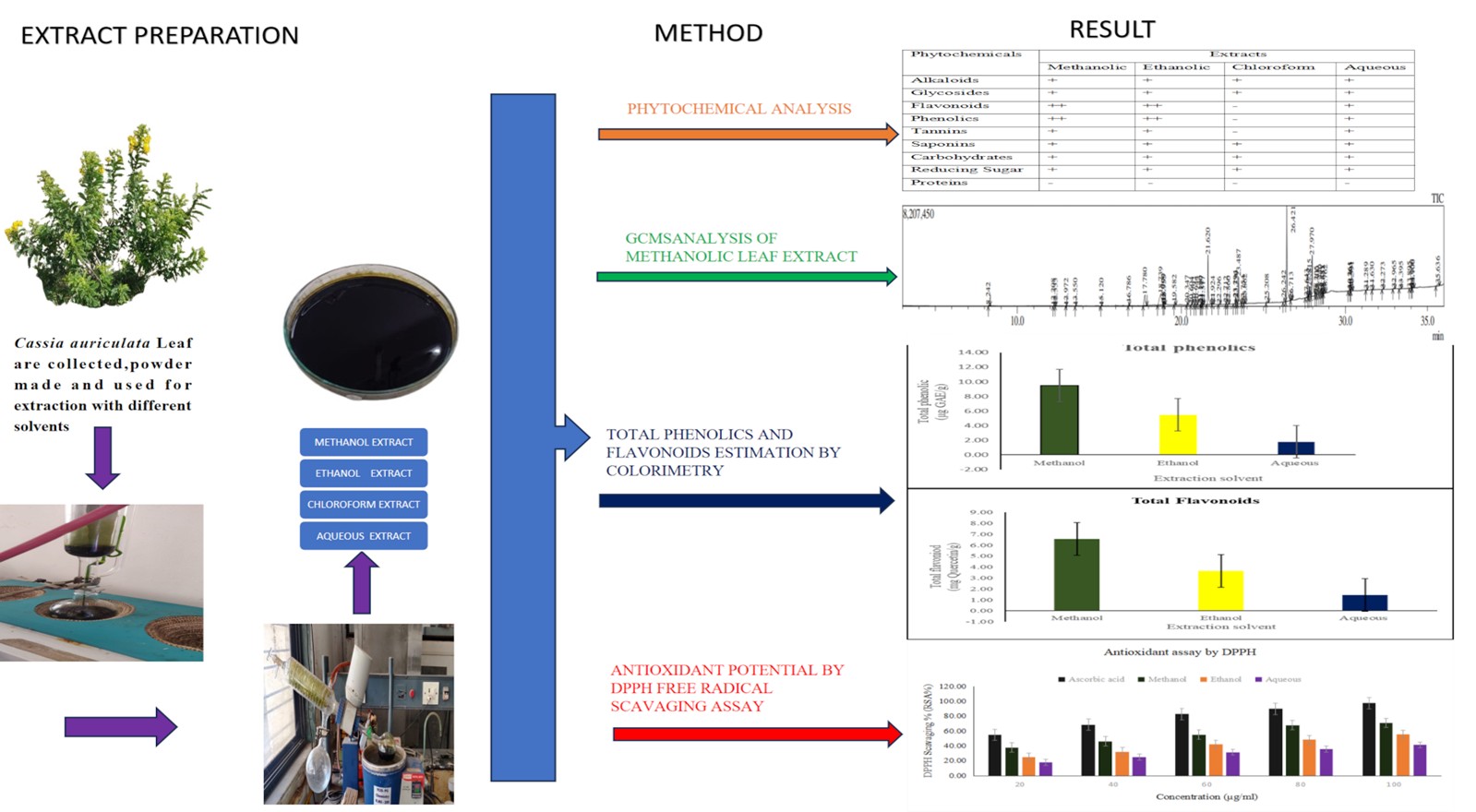
Background: This research aimed to investigate the phytochemical composition, quantify key bioactive compounds such as phenolics and flavonoids, analyze the secondary metabolite profile using GC-MS on methanolic leaf extracts, and evaluate the antioxidant capacity via the DPPH assay. Methodology: The Soxhlet extraction method is employed to obtain crude extracts of Cassia auriculata using solvents including ethanol, methanol, chloroform, and water. These extracts undertook qualitative analysis to detect various bioactive phytochemicals. The total phenolic and flavonoid concentrations were quantified. The methanolic leaf extract underwent phytochemical analysis using a gas chromatography-mass spectrometry (GC-MS) device, following established procedures. The antioxidant capacity of the methanolic leaf extracts was assessed by determining their ability to scavenge free radicals of 2,2-diphenyl-1-picrylhydrazyl. Results and Discussion: Initial phytochemical screening revealed the presence of various secondary metabolite groups. Out of all the solvent extracts assessed, the methanolic extract displayed the highest concentrations of phenolic and flavonoid compounds, measuring 9.48 ± 0.06 mg of Gallic acid equivalents per gram of extract and 6.56 ± 0.03 mg of Quercetin equivalents per gram of extract, respectively. GC-MS analysis of the methanolic extract identified 28 bioactive compounds with known pharmacological significance. Conclusion: The antioxidant activity, evaluated using the DPPH radical scavenging assay, demonstrated that the methanolic extract had the most potent radical scavenging effect among the extracts tested (IC50-48.96 µg/ml). These findings suggest that Cassia auriculata leaves extract is a promising source of natural antioxidants and bioactive compounds, supporting its traditional use in herbal medicine.
Downloads
References
Abraham A, Samuel S, Mathew L. Phytochemical analysis of Pathyashadangam kwath and its standardization by HPLC and HPTLC. J Ayurveda Integr Med, 11, 153–8 (2020) https://doi.org/10.1016/j.jaim.2017.10.011.
Rajagopal A, Rajakannu S. Cassia auriculata Linn. extracts induce apoptosis and cell cycle arrest of A549 lung cancer cell lines: An in vitro approach. South African Journal of Botany, 147, 275–85 (2022) https://doi.org/10.1016/j.sajb.2022.01.020.
Ouandaogo HS, Diallo S, Odari E, Kinyua J. Phytochemical Screening and GC-MS Analysis of Methanolic and Aqueous Extracts of Ocimum kilimandscharicum Leaves. ACS Omega, 8, 47560–72 (2023) https://doi.org/10.1021/acsomega.3c05554.
Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. Journal of Traditional and Complementary Medicine, 7, 234–44 (2017) https://doi.org/10.1016/j.jtcme.2016.05.006.
Deshpande S, Kewatkar SM, Paithankar VV. In-vitro antioxidant activity of different fraction of roots of Cassia auriculata Linn. Drug Invention Today, 5, 164–8 (2013) https://doi.org/10.1016/j.dit.2013.05.006.
Murugan M, Murugan T, Wins Ja. Antimicrobial Activity and Phytochemical Constituents of Leaf Extracts of Cassia auriculata. Indian J Pharm Sci, 75, 122 (2013) https://doi.org/10.4103/0250-474x.113546.
Kumar RS, Ponmozhi M, Viswanathan P, Nalini N. Effect of Cassia auriculata leaf extract on lipids in rats with alcoholic liver injury. Asia Pac J Clin Nutr, 11, 157–63 (2002) https://doi.org/10.1046/j.1440-6047.2002.00286.x.
Padmalochana K. Anticancer (liver cancer cell lines) and antioxidant activity of Cassia auriculata flower extract from acetone and methanol solvents. J. Drug Delivery Ther., 8, 274–8 (2018) https://doi.org/10.22270/jddt.v8i6-s.2130.
Vedavathy S, Rao KN. Antipyretic activity of six indigenous medicinal plants of Tirumala Hills, Andhra Pradesh, India. Journal of Ethnopharmacology, 33, 193–6 (1991) https://doi.org/10.1016/0378-8741(91)90178-g.
Devi PU, Selvi S, Suja S, Selvam K, Chinnaswamy P. Antidiabetic and Hypolipidemic Effect of Cassia auriculata in Alloxan Induced Diabetic Rats. IMR Press, 2, 601–7 (Invalid date) https://doi.org/10.3923/ijp.2006.601.607.
Esakkirajan M, Prabhu NM, Manikandan R, Beulaja M, Prabhu D, Govindaraju K, Thiagarajan R, Arulvasu C, Dhanasekaran G, Dinesh D, Babu G. Apoptosis mediated anti-proliferative effect of compound isolated from Cassia auriculata leaves against human colon cancer cell line. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 127, 484–9 (2014) https://doi.org/10.1016/j.saa.2014.02.073.
Bargah RK, Kushwaha A, Tirkey A, Hariwanshi B. In Vitro Antioxidant and Antibacterial Screening of flowers Extract from Cassia auriculata Linn. Rese. Jour. of Pharm. and Technol., 13, 2624 (2020) https://doi.org/10.5958/0974-360x.2020.00466.7.
Ismail HF, Hashim Z, Soon WT, Rahman NSA, Zainudin AN, Majid FAA. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. Journal of Traditional and Complementary Medicine, 7, 452–65 (2017) https://doi.org/10.1016/j.jtcme.2016.12.006.
Salma B, Janhavi P, Muthaiah S, Veeresh P, Santhepete Nanjundaiah M, Divyashree S, Serva Peddha M. Ameliorative Efficacy of the Cassia auriculata Root Against High-Fat-Diet + STZ-Induced Type-2 Diabetes in C57BL/6 Mice. ACS Omega, 6, 492–504 (2021) https://doi.org/10.1021/acsomega.0c04940.
Monisha M, Sowmiya M, Ragunathan R, Johney J. Extraction of Bio Active Compounds from Cassia Auriculata Pods and Leaves and its Medicinal Uses. Int.J.Curr.Microbiol.App.Sci, 6, 425–34 (2017) https://doi.org/10.20546/ijcmas.2017.608.056.
Patil S, S. Salve P, S. Phatak R, D. Chivate N. Quantitative Estimation of Total Phenolic, Total Flavonoid content and Assessment of In-Vitro Antioxidant Capacity of Psidium guajava L. Leaves Extracts. RJPT, 1028–32 (2023) https://doi.org/10.52711/0974-360x.2023.00172.
Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pacific Journal of Tropical Biomedicine, 4, S359–67 (2014) https://doi.org/10.12980/apjtb.4.2014c1030.
Kathirvel A, Sujatha V. Phytochemical studies, antioxidant activities and identification of active compounds using GC–MS of Dryopteris cochleata leaves. Arabian Journal of Chemistry, 9, S1435–42 (2016) https://doi.org/10.1016/j.arabjc.2012.03.018.
Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: An overview. International journal of chemical studies, 8, 603–8 (2020).
Esmat AU, Mittapally S, Begum S. GC-MS Analysis of Bioactive Compounds and Phytochemical Evaluation of the Ethanolic Extract of Gomphrena globosa L. Flowers. Journal of Drug Delivery and Therapeutics, 10, 53–8 (2020) https://doi.org/10.22270/jddt.v10i2.3914.
Yamin, Ruslin, Mistriyani, Sabarudin, Ihsan S, Armadany FI, Sahumena MH, Fatimah WON. Determination of total phenolic and flavonoid contents of jackfruit peel and in vitro antiradical test. Food Res., 5, 84–90 (2020) https://doi.org/10.26656/fr.2017.5(1).350.
Chambhare MR, Kadam NS, Doshi P, Kate SL, Nikule HA, Nikam TD. Anti-sickling potential and bioactive metabolites from edible oil-yielding crop Guizotia abyssinica (L.f.) Cass. Not Sci Biol, 17, 12164 (2025) https://doi.org/10.55779/nsb17112164.
Agrawal M, Arora S, Lahange P, Kshirsagar N, Government College of Pharmacy, Near Thiba Palace, Government Polytechnic Campus, Ratnagiri-415612, Maharashtra, India. Phytochemical Screening and Evaluation of Antioxidant Activity of hydroalcoholic extract of Justicia procumbans leaf. J. Ayu. Her. Med., 7, 41–5 (2021) https://doi.org/10.31254/jahm.2021.7109.
More R, Sarwade J, Kakade V, Daripkar O, Giri G, Markad G. Demography of Two Fishes Xenentodon cancila (Hamilton, 1822) and Hyporhamphus limbatus (Valenciennes, 1847) From Ujani Reservoir (Maharashtra, India) Facing Multiple Threats. (2025) https://doi.org/10.1111/lre.70017.
Heinrich M, Mah J, Amirkia V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules, 26, 1836 (2021) https://doi.org/10.3390/molecules26071836.
Maugeri A, Lombardo GE, Cirmi S, Süntar I, Barreca D, Laganà G, Navarra M. Pharmacology and toxicology of tannins. Arch Toxicol, 96, 1257–77 (2022) https://doi.org/10.1007/s00204-022-03250-0.
Pizzi A. Tannins medical / pharmacological and related applications: A critical review. Sustainable Chemistry and Pharmacy, 22, 100481 (2021) https://doi.org/10.1016/j.scp.2021.100481.
Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports, 24, e00370 (2019) https://doi.org/10.1016/j.btre.2019.e00370.
Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, Emwas A-H, Jaremko M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules, 25, 5243 (2020) https://doi.org/10.3390/molecules25225243.
Mazumder K, Nabila A, Aktar A, Farahnaky A. Bioactive Variability and In Vitro and In Vivo Antioxidant Activity of Unprocessed and Processed Flour of Nine Cultivars of Australian lupin Species: A Comprehensive Substantiation. Antioxidants (Basel), 9, 282 (2020) https://doi.org/10.3390/antiox9040282.
Shah AA, Gupta A, Rehman A-U-, Kapoor S, Kaur H, Rohilla B, Rashmi K, Bajpai A. GC-MS Analysis of Phytoactive Compounds, Antioxidant and Antibacterial Activity of Citrullus lanatus Seeds. Biomed. Pharmacol. J., 16, 73–86 (2023) https://doi.org/10.13005/bpj/2589.
Kumar P, Sati SC. Chemical composition, antioxidant and antimicrobial activities of Himalayan Fraxinus micrantha Lingelsh leaf extract. Natural Product Research, 35, 3519–23 (2021) https://doi.org/10.1080/14786419.2019.1710706.
Shelke DB, Tayade S, Gawande P, Sonawane HB. GC-MS analysis and antioxidant potential of wild underutilized medicinally important legume, velvet bean (Mucuna pruriens L. DC.). Notulae Scientia Biologicae, 14, 11098–11098 (2022).
Shetty A, Biradar PM. Analysis of different bioactive compounds in the tissue of the epigeic earthworm, Eisenia fetida. JoBAZ, 85, 28 (2024) https://doi.org/10.1186/s41936-024-00381-x.
Cupido M, De-Nova A, Guerrero-González ML, Pérez-Vázquez FJ, Méndez-Rodríguez KB, Delgado-Sánchez P. GC-MS analysis of phytochemical compounds of Opuntia megarrhiza (Cactaceae), an endangered plant of Mexico. PeerJ Organic Chemistry, 4, e5 (2022) https://doi.org/10.7717/peerj-ochem.5.
Soni K, Maurya P, Singh S. Bioactive compounds from fresh water green macro algae of Ganga water. J Pharmacogn Phytochem, 12, 369–85 (2023) https://doi.org/10.22271/phyto.2023.v12.i5d.14750.
Akter R, Maknun Fariha L, Halder S, Sharmin S, Sabet Taki E, Kabir Lihu I, Hamja Tipu A, Rubaiyat Muntasir Meem MM, Alam Ripa F, Sharmin S. GC‐MS‐employed Phytochemical Characterization and Anticancer, Antidiabetic, and Antioxidant Activity Screening of Lagerstroemia Thorelli. Chemistry & Biodiversity, 21, e202400999 (2024) https://doi.org/10.1002/cbdv.202400999.
Godara P, Dulara BK, Barwer N, Chaudhary NS. Comparative GC-MS Analysis of Bioactive Phytochemicals from Different Plant Parts and Callus of Leptadenia reticulata Wight and Arn. PJ, 11, 129–40 (2019) https://doi.org/10.5530/pj.2019.1.22.
Krishnamoorthy K, Subramaniam P. Phytochemical Profiling of Leaf, Stem, and Tuber Parts of Solena amplexicaulis (Lam.) Gandhi Using GC-MS. Int Sch Res Notices, 2014, 567409 (2014) https://doi.org/10.1155/2014/567409.
Olivia NU, Goodness UC, Obinna OM. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Futur J Pharm Sci, 7, 59 (2021) https://doi.org/10.1186/s43094-021-00208-4.
Katz DH, Marcelletti JF, Khalil MH, Pope LE, Katz LR. Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex. Proc. Natl. Acad. Sci. U.S.A., 88, 10825–9 (1991) https://doi.org/10.1073/pnas.88.23.10825.
Tyagi T, Agarwal M. GC-MS Analysis of invasive aquatic weed, Pistia stratiotes l. and Eichhornia crassipes (mart.) solms. Int J Curr Pharm Sci, 9, 111 (2017) https://doi.org/10.22159/ijcpr.2017.v9i3.19970.
Khan IH, Javaid A. hexanesoluble bioactive components of leaf extract of quinoa. The JAPS, 32, 609–14 (2022) https://doi.org/10.36899/japs.2022.2.0461.
Mustanir M, Nurdin N, Ginting B. Antioxidant Activity and Phytochemical Identification of Annona Squamosa Leaves Methanolic Extracts. PJ, 13, 1746–50 (2021) https://doi.org/10.5530/pj.2021.13.225.
Alghamdi AH, Khalid A, Ahmed AAE, Abdalgadir H, Bashir M, Abdalla AN, Ashgar SS, Alsaid HM, Oraiby ME. Therapeutic potential of Buddleja Polystachya Fresen (stem and leaves) extracts: antimicrobial and cytotoxic properties for ocular disease management. Discov Onc, 15, 282 (2024) https://doi.org/10.1007/s12672-024-01138-2.
Savalia V, Padariya J, Koshiya T, Pandya D. Phytochemical Investigation of Alysicarpus vaginalis by Gas Chromatography-mass Spectrometry and High-performance Thin-layer Chromatography. Scopus Indexed, 17, 7233–41 (2024) https://doi.org/10.37285/ijpsn.2024.17.2.4.
Sharma RK, Sharma N, Kumar U, Samant SS. Antioxidant properties, phenolics and flavonoids content of some economically important plants from North-west Indian Himalaya. Natural Product Research, 36, 1565–9 (2022) https://doi.org/10.1080/14786419.2021.1881959.
Mahato G, Banerjee N. Phytochemical analysis and DPPH antioxidant activity of two traditionally used plants occurring at purulia district of West Bengal, India. IJPSR, 8, (2017) https://doi.org/10.13040/ijpsr.0975-8232.8(12).5315-19.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Abhishek M Ranaware, Savita P. Nalawade

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















