Fabrication and study of release kinetics of moxifloxacin and dexamethasone loaded nanostructured lipid carrier system for ocular drug delivery
DOI:
https://doi.org/10.69857/joapr.v13i3.1162Keywords:
Nanostructured lipid carriers (NLCs), Ocular drug delivery, Moxifloxacin, Dexamethasone, Release KineticsAbstract
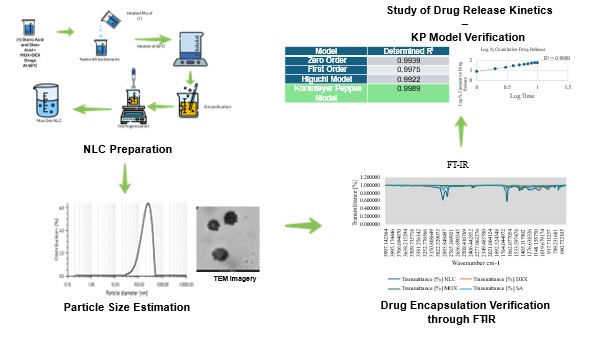
Background: The combination of moxifloxacin hydrochloride (MOX) and dexamethasone sodium phosphate (DEX) is widely available in the conventional commercial market for treating ocular infections and inflammations. Traditional ocular delivery systems are inferior to nanostructured lipid carriers (NLCs) due to their poor drug bioavailability, rapid tear drainage, and limited drug penetration. In contrast, NLCs offer sustained release, enhanced corneal absorption, and improved drug stability. Thus, the research aims to develop moxifloxacin and dexamethasone-loaded NLC for effective drug release. Methodology: In this study, a combination of MOX and DEX drugs was loaded in an NLC. The NLC was prepared using standard methods and evaluated for characteristic properties, including particle size (PS), polydispersity index (PDI), entrapment efficiency (EE), and drug loading (DL), as well as drug encapsulation and in vitro studies. Results and Discussion: The optimized formulation of NLC possessed a particle size of 190.58 nm and a polydispersity index of 26.7%. The fabricated drug exhibited a KP model release kinetics, indicating that drug release occurred via a combination of diffusion and polymer reaction. The NLC also exhibited a PDI of 26.7%, indicating a moderately uniform particle size distribution, which further suggests a consistent particle size, an acceptable characteristic for nano-carrier systems. The FT-IR analysis revealed optimal encapsulation of drugs inside the lipids, thereby achieving the desired objectives of drug fabrication. Conclusion: The formulated NLC has a particle size that falls within the ideal range for a smooth surface in commercial NLC formulations. Additionally, the prepared NLCs' adherence to the KP model underscores their potential as an advanced drug delivery system.
Downloads
References
Bharathi Mj, Amuthan M, Viswanathan S, Ramesh S, Ramakrishnan R. Prevalence of bacterial pathogens causing ocular infections in South India. Indian J Pathol Microbiol, 53, 281 (2010) https://doi.org/10.4103/0377-4929.64336.
Kurmi OP, Adhikari TB, Tyagi SK, Kallestrup P, Sigsgaard T. Addressing air pollution in India: Innovative strategies for sustainable solutions. Indian J Med Res, 160, 1–5 (2024) https://doi.org/10.25259/IJMR_691_2024.
John TJ, Cherian T, Raghupathy P. Haemophilus influenzae disease in children in India: a hospital perspective: The Pediatric Infectious Disease Journal, 17, S169–71 (1998) https://doi.org/10.1097/00006454-199809001-00015.
Mohanasundaram AS, Gurnani B, Kaur K, Manikkam R. Madras eye outbreak in India: Why should we foster a better understanding of acute conjunctivitis? Indian Journal of Ophthalmology, 71, 2298–9 (2023) https://doi.org/10.4103/IJO.IJO_3317_22.
Shah S, Wozniak RAF. Staphylococcus aureus and Pseudomonas aeruginosa infectious keratitis: key bacterial mechanisms that mediate pathogenesis and emerging therapeutics. Front. Cell. Infect. Microbiol., 13, 1250257 (2023) https://doi.org/10.3389/fcimb.2023.1250257.
Satpathy G, Behera H, Ahmed N. Chlamydial eye infections: Current perspectives. Indian J Ophthalmol, 65, 97 (2017) https://doi.org/10.4103/ijo.IJO_870_16.
Michalik M, Samet A, Podbielska-Kubera A, Savini V, Międzobrodzki J, Kosecka-Strojek M. Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: a review. Ann Clin Microbiol Antimicrob, 19, 26 (2020) https://doi.org/10.1186/s12941-020-00367-x.
Drago L. Topical Antibiotic Therapy in the Ocular Environment: The Benefits of Using Moxifloxacin Eyedrops. Microorganisms, 12, 649 (2024) https://doi.org/10.3390/microorganisms12040649.
McCulley JP, Caudle D, Aronowicz JD, Shine WE. Fourth-Generation Fluoroquinolone Penetration into the Aqueous Humor in Humans. Ophthalmology, 113, 955–9 (2006) https://doi.org/10.1016/j.ophtha.2006.01.061.
Lin MX, Guo L, Saldanha IJ, VanCourt S, Zeng J, Karakus S, Hessen M, Li G, Akpek EK. Dexamethasone Intracanalicular Insert for Clinically Significant Aqueous-Deficient Dry Eye. Ophthalmology, 131, 1033–44 (2024) https://doi.org/10.1016/j.ophtha.2024.03.010.
Zheng Q, Ge C, Li K, Wang L, Xia X, Liu X, Mehmood R, Shen J, Nan K, Chen W, Lin S. Remote-controlled dexamethasone-duration on eye-surface with a micelle-magnetic nanoparticulate co-delivery system for dry eye disease. Acta Pharmaceutica Sinica B, 14, 3730–45 (2024) https://doi.org/10.1016/j.apsb.2024.05.004.
L. Kiss E, Berkó S, Gácsi A, Kovács A, Katona G, Soós J, Csányi E, Gróf I, Harazin A, Deli MA, Budai-Szűcs M. Design and Optimization of Nanostructured Lipid Carrier Containing Dexamethasone for Ophthalmic Use. Pharmaceutics, 11, 679 (2019) https://doi.org/10.3390/pharmaceutics11120679.
Lakhani P, Patil A, Taskar P, Ashour E, Majumdar S. Curcumin-loaded Nanostructured Lipid Carriers for ocular drug delivery: Design optimization and characterization. Journal of Drug Delivery Science and Technology, 47, 159–66 (2018) https://doi.org/10.1016/j.jddst.2018.07.010.
Khan S, Sharma A, Jain V. An Overview of Nanostructured Lipid Carriers and its Application in Drug Delivery through Different Routes. Adv Pharm Bull, 13, 446–60 (2023) https://doi.org/10.34172/apb.2023.056.
Baig MS, Karade SK, Ahmad A, Khan MohdA, Haque A, Webster TJ, Faiyazuddin Md, Al-Qahtani NH. Lipid-based nanoparticles: innovations in ocular drug delivery. Front. Mol. Biosci., 11, 1421959 (2024) https://doi.org/10.3389/fmolb.2024.1421959.
Yan T, Ma Z, Liu J, Yin N, Lei S, Zhang X, Li X, Zhang Y, Kong J. Thermoresponsive GenisteinNLC-dexamethasone-moxifloxacin multi drug delivery system in lens capsule bag to prevent complications after cataract surgery. Sci Rep, 11, 181 (2021) https://doi.org/10.1038/s41598-020-80476-x.
Thapa C, Ahad A, Aqil Mohd, Imam SS, Sultana Y. Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box–Behnken design. Journal of Drug Delivery Science and Technology, 44, 431–9 (2018) https://doi.org/10.1016/j.jddst.2018.02.003.
Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharmaceutical Journal, 29, 999–1012 (2021) https://doi.org/10.1016/j.jsps.2021.07.015.
Patel D. Nanostructured Lipid Carriers (NLC)-Based Gel for Topical Delivery of Aceclofenac: Preparation, Characterization and In Vivo Evaluation. Sci. Pharm., 80, 749–64 (2012) https://doi.org/10.3797/scipharm.1202-12.
Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv Pharm Bull, 10, 150–65 (2020) https://doi.org/10.34172/apb.2020.021.
Hsueh Y-S, Shyong Y-J, Yu H-C, Jheng S-J, Lin S-W, Wu H-L, Tsai J-C. Nanostructured Lipid Carrier Gel Formulation of Recombinant Human Thrombomodulin Improve Diabetic Wound Healing by Topical Administration. Pharmaceutics, 13, 1386 (2021) https://doi.org/10.3390/pharmaceutics13091386.
Varela-Fernández R, García-Otero X, Díaz-Tomé V, Regueiro U, López-López M, González-Barcia M, Isabel Lema M, Javier Otero-Espinar F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. European Journal of Pharmaceutics and Biopharmaceutics, 172, 144–56 (2022) https://doi.org/10.1016/j.ejpb.2022.02.010.
Deep A, Kumar M, Pahwa R, Gupta S, Bhatt S, Kumari B, Upadhyay PK, Pandurangan A. Formulation and in vivo pharmacodynamics studies of nanostructured lipid carriers for topical delivery of bifonazole. actapharm, 59, 559 (2021) https://doi.org/10.23893/1307-2080.APS.05902.
Pezeshki A, Ghanbarzadeh B, Mohammadi M, Fathollahi I, Hamishehkar H. Encapsulation of Vitamin A Palmitate in Nanostructured Lipid Carrier (NLC)-Effect of Surfactant Concentration on the Formulation Properties. Advanced Pharmaceutical Bulletin; eISSN 2251-7308, (2014) https://doi.org/10.5681/APB.2014.083.
Gilani SJ, Jumah MNB, Zafar A, Imam SS, Yasir M, Khalid M, Alshehri S, Ghuneim MM, Albohairy FM. Formulation and Evaluation of Nano Lipid Carrier-Based Ocular Gel System: Optimization to Antibacterial Activity. Gels, 8, 255 (2022) https://doi.org/10.3390/gels8050255.
Lakhani P, Patil A, Taskar P, Ashour E, Majumdar S. Curcumin-loaded Nanostructured Lipid Carriers for ocular drug delivery: Design optimization and characterization. Journal of Drug Delivery Science and Technology, 47, 159–66 (2018) https://doi.org/10.1016/j.jddst.2018.07.010.
Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics, 13, 531 (2021) https://doi.org/10.3390/pharmaceutics13040531.
Gómez-Lázaro L, Martín-Sabroso C, Aparicio-Blanco J, Torres-Suárez AI. Assessment of In Vitro Release Testing Methods for Colloidal Drug Carriers: The Lack of Standardized Protocols. Pharmaceutics, 16, 103 (2024) https://doi.org/10.3390/pharmaceutics16010103.
Ahalwat S, Bhatt DC. Development of novel lipid matrix for improved sustained release effect of a hydrophilic drug via response surface methodology. Journal of Drug Delivery Science and Technology, 67, 102993 (2022) https://doi.org/10.1016/j.jddst.2021.102993.
Pinheiro M, Ribeiro R, Vieira A, Andrade F, Reis S. Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. DDDT, Volume 10, 2467–75 (2016) https://doi.org/10.2147/DDDT.S104395.
Deshkar SS, Jadhav MS, Shirolkar SV. Development of Carbamazepine Nanostructured Lipid Carrier Loaded Thermosensitive Gel for Intranasal Delivery. Adv Pharm Bull, 11, 150–62 (2020) https://doi.org/10.34172/apb.2021.016.
Azhar Shekoufeh Bahari L, Hamishehkar H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv Pharm Bull, 6, 143–51 (2016) https://doi.org/10.15171/apb.2016.021.
Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomedicine & Pharmacotherapy, 103, 598–613 (2018) https://doi.org/10.1016/j.biopha.2018.04.055.
Nguyen VH, Thuy VN, Van TV, Dao AH, Lee B-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano, 8, 100064 (2022) https://doi.org/10.1016/j.onano.2022.100064.
Gade S, Patel KK, Gupta C, Anjum MdM, Deepika D, Agrawal AK, Singh S. An Ex Vivo Evaluation of Moxifloxacin Nanostructured Lipid Carrier Enriched In Situ Gel for Transcorneal Permeation on Goat Cornea. Journal of Pharmaceutical Sciences, 108, 2905–16 (2019) https://doi.org/10.1016/j.xphs.2019.04.005.
Joshi PH, Youssef AAA, Ghonge M, Varner C, Tripathi S, Dudhipala N, Majumdar S. Gatifloxacin Loaded Nano Lipid Carriers for the Management of Bacterial Conjunctivitis. Antibiotics, 12, 1318 (2023) https://doi.org/10.3390/antibiotics12081318.
Mall J, Naseem N, Haider MdF, Rahman MA, Khan S, Siddiqui SN. Nanostructured lipid carriers as a drug delivery system: A comprehensive review with therapeutic applications. Intelligent Pharmacy, S2949866X24001023 (2024) https://doi.org/10.1016/j.ipha.2024.09.005.
Viegas C, Patrício AB, Prata JM, Nadhman A, Chintamaneni PK, Fonte P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics, 15, 1593 (2023) https://doi.org/10.3390/pharmaceutics15061593.
Nagaich U, Gulati N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv. and Transl. Res., 6, 289–98 (2016) https://doi.org/10.1007/s13346-016-0291-1.
Youssef AAA, Thakkar R, Senapati S, Joshi PH, Dudhipala N, Majumdar S. Design of Topical Moxifloxacin Mucoadhesive Nanoemulsion for the Management of Ocular Bacterial Infections. Pharmaceutics, 14, 1246 (2022) https://doi.org/10.3390/pharmaceutics14061246.
Youssef AAA, Dudhipala N, Majumdar S. Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications. IJN, Volume 17, 2283–99 (2022) https://doi.org/10.2147/IJN.S360740.
Han H, Li S, Xu M, Zhong Y, Fan W, Xu J, Zhou T, Ji J, Ye J, Yao K. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Advanced Drug Delivery Reviews, 196, 114770 (2023) https://doi.org/10.1016/j.addr.2023.114770.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Narayan Hemnani, Preeti K. Suresh

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















