Development and validation of stability indicating RP-HPLC method for nebivolol by using the DOE approach
DOI:
https://doi.org/10.69857/joapr.v13i3.1062Keywords:
Nebivolol, RP-HPLC, Stability-Indicating Method, Forced Degradation, Validation, Quality ControlAbstract
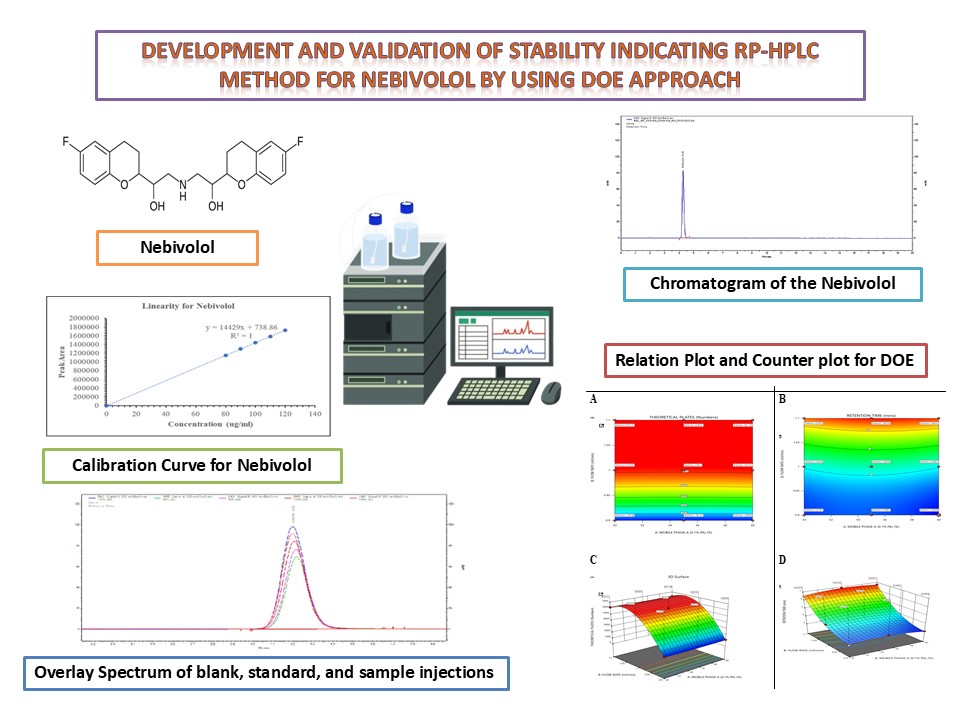
Background: Nebivolol (NBV), classified as a third-generation β1-adrenergic receptor antagonist, is commonly prescribed for managing hypertension. Accurate and prompt quantification of NBV in bulk materials and pharmaceutical formulations is crucial for quality assurance. This research focuses on developing and validating a stability-indicating RP-HPLC method for the quantification of NBV, with an emphasis on sensitivity, precision, accuracy, and robustness. Methodology: A stability-indicating reversed-phase high-performance liquid chromatography (RP-HPLC) method was established using an Agilent 1260 Infinity II HPLC system equipped with a Diode Array Detector (DAD). The chromatographic analysis was conducted on an Agilent Zorbax Bonus RP column (250 × 4.6 mm, 5 µm). The mobile phase comprised acetonitrile and 0.1% perchloric acid in a 55:45 (v/v) ratio, delivered at a flow rate of 1 mL/min. Detection was performed at a wavelength of 282 nm. The method underwent validation according to the guidelines provided by the International Council for Harmonisation (ICH), including assessments of linearity, precision, accuracy, robustness, and forced degradation studies. Results and Discussion: This method demonstrated improved sensitivity, shorter run time (retention time of 4.22 min), and high precision. Forced degradation studies confirmed Nebivolol’s instability under alkaline (15.94%) and oxidative (8.57%) conditions, highlighting the method’s stability-indicating capability. The method also gives robust linearity across the concentration range of 80–120 µg/mL, with a correlation coefficient (r²) of 1.00. The limits of detection (LOD) and quantification (LOQ) were determined to be 0.55 µg/mL and 1.61 µg/mL, respectively. Conclusion: The proposed RP-HPLC method proved to be reliable, precise, and stability-indicating, making it a valuable tool for the quality control and stability assessment of Nebivolol formulations in pharmaceutical settings.
Downloads
References
Rajoriya V, Kashaw V. RP-HPLC method for the simultaneous estimation of nebivolol hydrochloride and valsartan. Anal. Chem. Lett., 7(4), 520-530 (2017) https://doi.org/10.1080/22297928.2017.1362994.
Chaudhari B, Daniel K. A validated RP-HPLC method for simultaneous estimation of tizanidine hydrochloride and nimesulide in bulk and pharmaceutical formulation. Res. J. Pharm. Technol., 13(9), 4207-4212 (2020) https://doi.org/10.5958/0974-360X.2020.00743.X.
Reddy CA, Zeeyauddin K, Rajendra Y. Method development and validation for nebivolol and valsartan by RP-HPLC method. Eur. J. Biomed., 9(1), 138-143 (2022) https://doi.org/10.13040/IJPSR.0975-8232.
Khan SA, Rehman S, Nabi B, Iqubal A, Nehal N, Fahmy UA, Kotta S, Baboota S, Md S, Ali J. Boosting the brain delivery of Atazanavir through nanostructured lipid carrier-based approach for mitigating neuroaids. Pharmaceutics, 12(11), 1059 (2020) https://doi.org/10.3390/pharmaceutics12111059.
Ghereghlou M, Afshar J, Mousavi SH. Simultaneous determination of potassium sorbate and sodium benzoate in processed food samples available in Mashhad market with a validated method using HPLC-DAD. Iran. J. Anal. Chem., 10(2), (2024) https://doi.org/110.30473/ijac.2024.70436.1286.
Verma K, Rastogi S, Dahiya M, Singh GP, Chauhan J, Tomar P, Kumar S. Development and validation of RP-HPLC method using UV detection for simultaneous quantification of amlodipine besylate and nebivolol hydrochloride in fixed-dose combination tablets. Res. J. Pharm. Technol., 17(2), 523-528 (2024) https://doi.org/10.52711/0974-360X.2024.00082.
Vashistha VK, Kumar A. Stereochemical facets of clinical β‐blockers: An overview. Chirality, 32(5), 722-735 (2020) https://doi.org/10.1002/chir.23200.
Vaditake KT, Shirkhedkar AA. Simultaneous estimation of nebivolol hydrochloride and amlodipine besylate in human plasma employing an innovative HPLC chromatographic method. Future J. Pharm. Sci., 10(1), 142 (2024) https://doi.org/10.1186/s43094-024-00716-z.
Haque SM. Box–Behnken experimental design for optimizing the HPLC method to determine hydrochlorothiazide in pharmaceutical formulations and biological fluid. J. Mol. Liq., 352, 118708 (2022) https://doi.org/10.1016/j.molliq.2022.118708.
Latha M, et al. Design, optimization, and justification of RP-HPLC process for the resolvation of azelnidipine in bulk plus in pharmaceutical components. Res. J. Pharm. Technol., 17(5), 2175-2179 (2024) https://doi.org/10.52711/0974-360X.2024.00342.
Dash RN, Habibuddin M, Sahoo A, Kothawade SN, Chaudhari MR, Mahadik KR. Factorial approach for the development of stability indicating HPLC assay of recombinant human insulin: Application to its stability study. Curr. Pharm. Anal., 9(3), 318-329 (2013) https://doi.org/10.2174/1573412911309030010.
Sowjanya G, Parimala PD, Oindrila M, Praveen Kumar S. Development and validation of a new derivative UV spectrophotometric method for simultaneous quantification of tizanidine and aceclofenac in tablets. Res. J. Pharm. Technol., 13(2), 569-574 (2020) https://doi.org/10.5958/0974-360X.2020.00107.9.
Labhade SD, Chaudhari SR, Saudagar RB. Development and validation of RP-HPLC method for simultaneous determination of diclofenac sodium and tizanidine hydrochloride in bulk and tablet formulation. J. Anal. Pharm. Res., 7(2), 244-247 (2018) https://doi.org/10.15406/japlr.2018.07.00233.
Rani R, Chaudhari L, Dhanorya D, Ahirwar D, Kori S, Ahirwar V, et al. Development of stability indicating RP-HPLC method for tizanidine hydrochloride in bulk drug and pharmaceutical dosage form. J. Drug Deliv. Ther., 13(3), 131-137 (2023) http://dx.doi.org/10.22270/jddt.v13i3.5780.
Vaditake KT, Shirkhedkar AA. Simultaneous estimation of nebivolol hydrochloride and amlodipine besylate in human plasma employing an innovative HPLC chromatographic method. Future J. Pharm. Sci., 10(1), 142 (2024) https://doi.org/10.1186/s43094-024-00716-z.
Zaman M, Hanif M, Khan NU, Mahmood A, Qaisar MN, Ali H. Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of tizanidine HCl and meloxicam in rabbit's plasma. Acta Chromatogr., 31(3), 173-178 (2019) https://doi.org/10.1556/1326.2018.00408.
Kothawade SN, Pande VV, Albhar SN, Bole SS, Raut KG, Wagh VS. A high-performance stability-indicating liquid chromatographic novel method for determining recombinant human erythropoietin in bulk and dosage form. Preprints, 1, 1-22 (2022) https://doi.org/10.20944/preprints202211.0577.v1.
Sundari MM, Surekha ML. Method development and validation of simultaneous estimation of alogliptin and pioglitazone in combined tablet dosage forms by RP-HPLC. J. Popul. Ther. Clin. Pharmacol., 29(3), 425-440 (2022) https://doi.org/10.53555/jptcp.v29i03.3779.
John LZ, Khalid M, Adeleye L. Optimization of antibiotic analysis in water by solid-phase extraction and high-performance liquid chromatography-mass spectrometry/mass spectrometry. Anal. Chim. Acta., 731, 32-39 (2012) https://doi.org/10.1016/j.aca.2012.04.021.
Annadi A, Shoheib RE, Mohamed A. Assessments of valsartan in the presence of nebivolol or amlodipine in solid formulations and its discriminative dissolution behavior via RP-HPLC and RP-UPLC methods. Egypt. J. Chem., 63(8), 2837-2851 (2020) https://doi.org/10.21608/ejchem.2020.18176.2116.
Pratap SNH, Ratna JV. Development and validation of a sensitive and rapid bioanalytical RP-HPLC method for the quantification of nebivolol hydrochloride in rat plasma. Curr. Trends Biotechnol. Pharm., 16(3s), 87-95 (2022) https://doi.org/10.5530/ctbp.2022.3s.67.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Anant Ghongade, Shruti Barot

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















