Development and validation of a stability-indicating RP-HPLC method for tizanidine hydrochloride using DOE
DOI:
https://doi.org/10.69857/joapr.v13i3.1057Keywords:
Tizanidine hydrochloride, RP-HPLC, Stability-indicating method, Design of Experiments (DOE), Forced degradation, Analytical validationAbstract
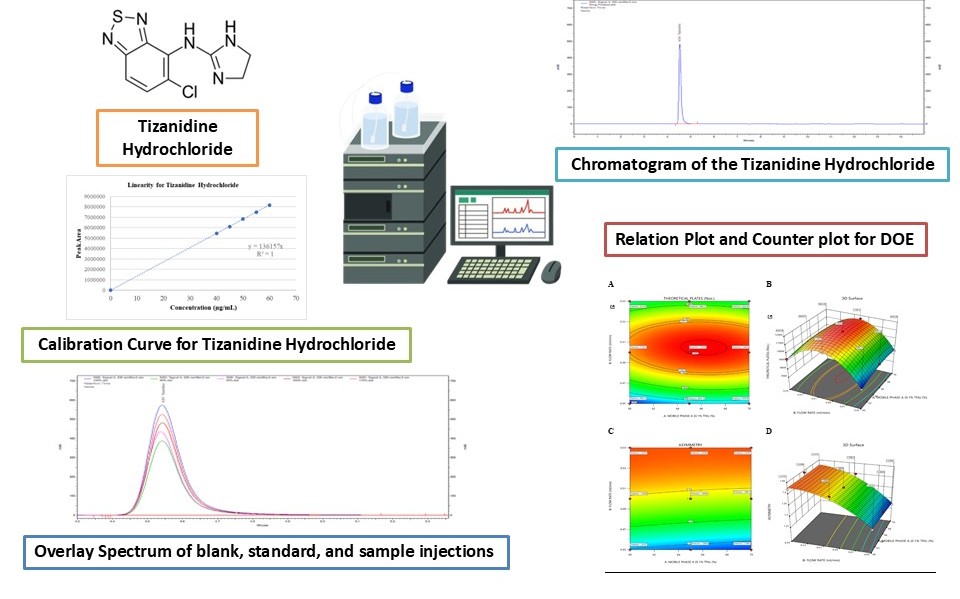
Background: Tizanidine hydrochloride (TIZ) is a centrally acting α2-adrenergic receptor agonist widely prescribed for managing spasticity. Given its therapeutic importance, a reliable, stability-indicating analytical method is crucial to ensure both the quality and regulatory compliance of Tizanidine hydrochloride. Existing RP-HPLC methods often lack robustness, sensitivity, or DoE optimization, highlighting the need for an improved approach. Methodology: A stability-indicating reverse-phase high-performance liquid chromatography (RP-HPLC) method with stability-indicating properties was developed and validated using a Design of Experiments (DoE) approach. A full factorial design was implemented, optimizing mobile phase composition and flow rate as key method variables. Chromatographic separation was achieved using an Agilent Zorbax Bonus RP column (250 × 4.6 mm, 5 µm) with a mobile phase of 0.1% trifluoroacetic acid (TFA) and acetonitrile (65:35 v/v) at a 0.5 mL/min flow rate. Detection was performed at 228 nm. The method was validated by ICH guidelines, evaluating parameters such as specificity, precision, accuracy, linearity, robustness, and forced degradation. Results and Discussion: The method demonstrated excellent linearity (r² = 1.00) across concentration levels ranging from 80% to 120% of the target concentration. The LOD and LOQ were 1.00 µg/mL and 3.04 µg/mL, respectively. High precision (%RSD < 2%) and accuracy (99–101% recovery) were observed. Forced degradation studies revealed notable degradation under oxidative (36.08%) and acidic (15.73%) conditions. Conclusion: The developed RP-HPLC method is precise, robust, and suitable for the routine quality control and stability assessment of Tizanidine hydrochloride in pharmaceutical formulations.
Downloads
References
Patel KY, Dedania ZR, Dedania RR, Patel U. QbD Approach to HPLC Method Development and Validation of Ceftriaxone Sodium. Future J. Pharm. Sci., 7, 1–10 (2021) https://doi.org/10.1186/s43094-021-00286-4.
Fegade BS, Mhatre AS, Munipalli VK, Magar HP, Thakur PP, Kumar A, Bhaskar V. Development and Validation of a Stability Indicating RP-HPLC Method for the Estimation of Deferiprone in its Capsule Dosage Form. Res. J. Pharm. Technol., 17(6), 2725-2731 (2024) https://doi.org/0.52711/0974-360X.2024.00427.
Lingareddygari SR, et al. Design of Experiments Approach for Method Development and Validation of Bilastine in Pure and Pharmaceutical Dosage Form Using RP-UFLC. Orient. J. Chem., 39(3), 736-745 (2023) http://dx.doi.org/10.13005/ojc/390325.
Chaudhari B, Daniel K. A Validated RP-HPLC Method for Simultaneous Estimation of Tizanidine Hydrochloride and Nimesulide in Bulk and Pharmaceutical Formulation. Res. J. Pharm. Technol., 13(9), 4207-4212 (2020) https://doi.org/10.5958/0974-360X.2020.00743.X.
Mital P, Charmy K, Vivek V. An Innovative Impurity Profiling of Avanafil Using LC and LC-MS/MS with In-Silico Toxicity Prediction. Arab. J. Chem., 13(8), 6493–6509 (2020) https://doi.org/10.1016/j.arabjc.2020.06.007.
Sowjanya G, Parimala PD, Oindrila M, Praveen Kumar S. Development and Validation of a New Derivative UV Spectrophotometric Method for Simultaneous Quantification of Tizanidine and Aceclofenac in Tablets. Res. J. Pharm. Technol., 13(2), 569-574 (2020) https://doi.org/10.5958/0974-360X.2020.00107.9.
Latha M, et al. Design, Optimization, and Justification of RP-HPLC Process for the Resolvation of Azelnidipine in Bulk Plus in Pharmaceutical Components. Res. J. Pharm. Technol., 17(5), 2175-2179 (2024) https://doi.org/10.52711/0974-360X.2024.00342.
Labhade SD, Chaudhari SR, Saudagar RB. Development and Validation of RP-HPLC Method for Simultaneous Determination of Diclofenac Sodium and Tizanidine Hydrochloride in Bulk and Tablet Formulation. J. Anal. Pharm. Res., 7(2), 244-247 (2018) https://doi.org/10.15406/japlr.2018.07.00233.
Dash RN, Habibuddin M, Sahoo A, Kothawade SN, Chaudhari MR, Mahadik KR. Factorial Approach for the development of stability indicating HPLC assay of recombinant human insulin: Application to its stability study. Curr. Pharm. Anal., 9(3), 318-329 (2013) https://doi.org/10.2174/1573412911309030010.
Rani R, Chaudhari L, Dhanorya D, Ahirwar D, Kori S, Ahirwar V, et al. Development of Stability Indicating RP-HPLC Method for Tizanidine Hydrochloride in Bulk Drug and Pharmaceutical Dosage Form. J. Drug Deliv. Ther., 13(3), 131-137 (2023) https://doi.org/10.22270/jddt.v13i3.5780.
Gope ER, Begum SM, Anisetti PP, Kasa GG, Eedarada VG, Nalli J, Thummidi RS. A Review of Principles, Applications, and Recent Developments in HPTLC and HPLC. J. Pharm. Insights Res., 2(6), 056–064 (2024) https://doi.org/10.69613/315vge42.
Rajesh R, Selvakumar K. Stability Indicating RP-HPLC Method Development and Validation for the Analysis of Tizanidine Hydrochloride in Bulk and Pharmaceutical Formulation. J. Pharm. Sci. Res., 12(9), 1162-1169 (2020) https://dx.doi.org/10.22159/ijpps.2024v16i4.50126.
Zaman M, Hanif M, Khan NU, Mahmood A, Qaisar MN, Ali H. Development and Validation of Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Tizanidine HCl and Meloxicam in Rabbit's Plasma. Acta Chromatogr., 31(3), 173-178 (2019) https://doi.org/10.1556/1326.2018.00408.
Ahirwar P, Khare B, Jain PK, Jain A, Khan R, Thakur B. Compressive Review on Role of ICH Guidelines in Registration of Pharmaceutical Products. Asian J. Dent. Health Sci., 2(3), 1-8 (2022) http://dx.doi.org/10.22270/ajdhs.v2i3.16.
Kothawade SN, Pande VV, Albhar SN, Bole SS, Raut KG, Wagh VS. A high-performance stability-indicating liquid chromatographic novel method for determining recombinant human erythropoietin in bulk and dosage form. Preprints, 1, 1-22 (2022) https://doi.org/10.20944/preprints202211.0577.v1.
Sundari MM, Surekha ML. Method Development and Validation of Simultaneous Estimation of Alogliptin and Pioglitazone in Combined Tablet Dosage Forms by RP-HPLC. J. Popul. Ther. Clin. Pharmacol., 29(3), 425-440 (2022) https://doi.org/10.53555/jptcp.v29i03.3779.
Haas CP, Biesenroth S, Buckenmaier S, van de Goor T, Tallarek U. Automated Generation of Photochemical Reaction Data by Transient Flow Experiments Coupled with Online HPLC Analysis. React. Chem. Eng., 5(5), 912–920 (2020) https://doi.org/10.1039/D0RE00066C.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Prasad Gunjal, Janki Patel

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















