Role of intrinsic and supplemented antioxidants in follicular fluid: a shield against oxidative stress in oocyte health and embryo development
DOI:
https://doi.org/10.69857/joapr.v13i3.1036Keywords:
Antioxidants, Coenzyme, Follicular fluid, Intrinsic, In Vitro FertilizationAbstract
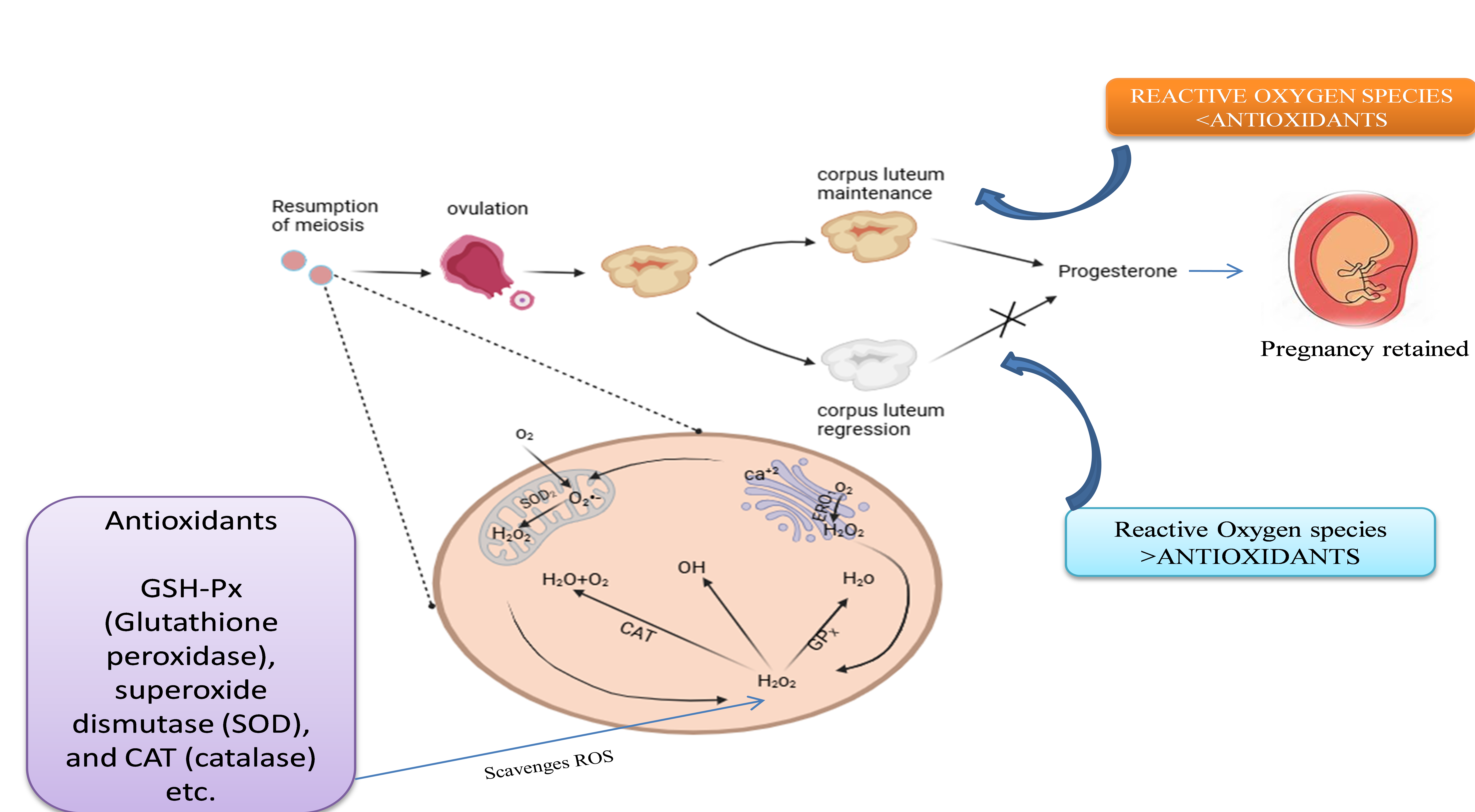
Background: In In Vitro Fertilization (IVF), a form of Assisted Reproductive Technologies (ART), the quality of oocytes and the successful development of embryos are crucial in determining the rate of fertility. The excessive presence of ROS (Reactive Oxygen Species) can cause oxidative stress, which negatively affects follicular fluid (FF) and oocyte maturation. Certain non-endogenous antioxidants, such as catalase, glutathione, and Superoxide Dismutase (SOD), are already present in Follicular fluid, which counterbalances these ROS and protects oocytes. Method: In addition to examining the possibility of exogenous supplements of antioxidants, such as vitamins C and E, and Coenzyme Q10 (CoQ10), this review investigates the function of these intrinsic antioxidants in maintaining oocyte health. Result: According to current in vivo and in vitro research findings done in mice, pigs, sheep, cows, and 18 patients in the age group(40±1), respectively, targeted antioxidant supplementation may enhance oocyte quality, embryo viability, and pregnancy outcomes. Conclusion: However, addressing individual heterogeneity in oxidative stress and optimizing dosage remains challenging. This review highlights how new antioxidant compounds and targeted interventions may enhance reproductive success by promoting cellular resilience in follicular fluid (FF). However, additional research into targeted antioxidant therapy in IVF is necessary.
Downloads
References
Gairola N, Chitme HR, Sircar R. Human follicular fluid, clinical use of proteomics analysis in identification of infertility. Indian J. Pharm. Educ. Res., 56(4), 917–922 (2022) https://doi.org/10.5530/ijper.56.4.146
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med., 9(12), e1001356 (2012). https://doi.org/10.1371/journal.pmed.1001356
Shi Y, Zhu H, Zhang Y, Ma X, Han X, Jiang X, Zhang Y. Premature ovarian insufficiency: A review on the role of oxidative stress and the application of antioxidants. Antioxidants, 12(7), 1563 (2023) https://doi.org/10.3390/antiox12071563
Pant P, Chitme HR, Sircar R, Prasad R, Prasad HO. Genome-wide association study for single nucleotide polymorphism associated with mural and cumulus granulosa cells of PCOS (polycystic ovary syndrome) and non-PCOS patients. Future J. Pharm. Sci., 9(1), 27 (2023) https://doi.org/10.1186/s43094-023-00475-3
Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol., 3, 28 (2005) https://doi.org/10.1186/1477-7827-3-28
Cao B, Qin J, Pan B, Qazi IH, Ye J, Fang Y, Zhou G. Oxidative stress and oocyte cryopreservation: Recent advances in mitigation strategies involving antioxidants. Cells, 11(22), 3573 (2022) https://doi.org/10.3390/cells11223573
Ishak GM, Feugang JM, Pechanova O, Pechan T, Peterson DG, Willard ST, Ryan PL, Gastal EL. Follicular‐fluid proteomics during equine follicle development. Mol. Reprod. Dev., 89(7), 298–311 (2022) https://doi.org/10.1002/mrd.23622
Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. The follicular fluid proteome and its role in oocyte quality and reproductive outcome. Reprod. Biol. Endocrinol., 7(1), 140 (2009) https://doi.org/10.1186/1477-7827-7-140
Wang Y, Li X, Zhang Z, Liu Y, Chen H. Unraveling the complexity of follicular fluid: Insights into its composition, function, and clinical implications. J. Ovarian Res., 17(1), 45 (2024) https://doi.org/10.1186/s13048-024-01551-9
Liang X, Wang Y, Zhang Z, Liu Y, Chen H. Oxidative stress and inflammatory markers in ovarian follicular fluid of patients with diminished ovarian reserve: A comparative study. J. Ovarian Res., 16(1), 123 (2023) https://doi.org/10.1186/s13048-023-01293-0
Agarwal A, Rana M, Qamar AY, Almekhlafi M, Arafa M, Henkel R. Role of reactive oxygen species in the pathophysiology of human reproduction. Reprod. Biol. Endocrinol., 20(1), 95 (2022) https://doi.org/10.1186/s12958-022-00952-4
Liang J, Gao Y, Feng Z, Zhang B, Na Z, Li D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol., 62, 102659 (2023) https://doi.org/10.1016/j.redox.2023.102659
Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov., 21(2), 115–140 (2022) https://doi.org/10.1038/s41573-021-00320-3
Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ. Reactive oxygen species signaling in the diabetic heart: emerging prospect for therapeutic targeting. Heart, 104(4), 293–299 (2018) https://doi.org/10.1136/heartjnl-2017-311448
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol., 10(1), 49 (2012) https://doi.org/10.1186/1477-7827-10-49
Goud PT, Goud AP, Diamond MP, Gonik B, Abu-Soud HM. Oxidative stress in ovarian follicular fluid compromises oocyte quality and embryonic development. Reprod. Sci., 29(2), 376–387 (2022) https://doi.org/10.1007/s43032-021-00566-2
Jeremic A, Vasiljevic M, Mikovic Z, Bukumiric Z, Simic P, Stanisavljevic T, Simic T, Djukic T. Oxidative homeostasis in follicular fluid and embryo quality—a pilot study. Int. J. Mol. Sci., 26(8), 3560 (2025) https://doi.org/10.3390/ijms26083560
Liu Y, Yu Z, Zhao S, Cheng L, Man Y, Gao X, Zhao H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet., 38(2), 471–477 (2021) https://doi.org/10.1007/s10815-020-02014-y
Moura MT, Lopes ACB, Souza MDCD, Ribeiro DP, Gomes MA, Alves MTSSB. The role of antioxidant supplementation in improving reproductive outcomes: A review of the current evidence. Antioxidants, 10(8), 1268 (2021) https://doi.org/10.3390/antiox10081268
Zarbakhsh S. Effect of antioxidants on preimplantation embryo development in vitro: A review. Zygote, 29(3), 179–193 (2021) https://doi.org/10.1017/S0967199420000660
Zhou Q, Li J, Wang Y, Chen X. Impact of oxidative stress on early embryonic development: Mechanisms and protective strategies. J. Reprod. Biol. Endocrinol., 21(1), 45 (2023) https://doi.org/10.1186/s12958-023-01055-2
Gairola N, Chitme HR, Sircar R. Systemic effects of clinical follicular fluid from polycystic ovary syndrome and non-polycystic ovary syndrome in female mice. Indian J. Exp. Biol., 62(3), 169–178 (2024). https://doi.org/10.56042/ijeb.v62i03.1910.
Drejza MA, Rylewicz K, Majcherek E, Gross-Tyrkin K, Mizgier M, Plagens-Rotman K, Wójcik M, Panecka-Mysza K, Pisarska-Krawczyk M, Kędzia W, Jarząbek-Bielecka G. Markers of oxidative stress in obstetrics and gynaecology—A systematic literature review. Antioxidants, 11(8), 1477 (2022) https://doi.org/10.3390/antiox11081477
Al-Saleh I, Coskun S, Al-Rouqi R, Al-Rajudi T, Eltabache C, Abduljabbar M, Al-Hassan S. Oxidative stress and DNA damage status in couples undergoing in vitro fertilization treatment. Reprod. Fertil., 2(2), 117–139 (2021) https://doi.org/10.1530/RAF-20-0062
Iman MM, Mohammed HH, Elmahdy AM. Clinical significance of oxidative stress biomarkers in the follicular fluid of women undergoing in vitro fertilization. J. Gynecol. Obstet., 8(1), 7–14 (2020) https://doi.org/10.11648/j.jgo.20200801.12
Oyawoye O. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod., 18(11), 2270–2274 (2003) https://doi.org/10.1093/humrep/deg450
Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil. Steril., 89(4), 912–921 (2008) https://doi.org/10.1016/j.fertnstert.2007.04.034
Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil. Steril., 81(4), 973–976 (2004) https://doi.org/10.1016/j.fertnstert.2003.11.021
Chen Y, Yang J, Zhang L. The impact of follicular fluid oxidative stress levels on the outcomes of assisted reproductive therapy. Antioxidants, 12(12), 2117 (2023) https://doi.org/10.3390/antiox12122117
Debbarh H, Louanjli N, Aboulmaouahib S, Jamil M, Ahbbas L, Kaarouch I, Sefrioui O, Cadi R. Antioxidant activities and lipid peroxidation status in human follicular fluid: age-dependent change. Zygote, 29(6), 490–494 (2021) https://doi.org/10.1017/S0967199421000241
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol., 10(1), 49 (2012) https://doi.org/10.1186/1477-7827-10-49
Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil. Steril., 77(6), 1184–1190 (2002) https://doi.org/10.1016/S0015-0282(02)03103-5
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res., 44(3), 280–287 (2008) https://doi.org/10.1111/j.1600-079X.2007.00524.x
da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J. Assist. Reprod. Genet., 35(5), 735–751 (2018) https://doi.org/10.1007/s10815-018-1143-3
Kokot I, Piwowar A, Jędryka M, Kratz EM. Is there a balance in oxidative-antioxidant status in blood serum of patients with advanced endometriosis? Antioxidants, 10(7), 1097 (2021) https://doi.org/10.3390/antiox10071097
Santulli P, Chouzenoux S, Fiorese M, Marcellin L, Lemarechal H, Millischer AE, Batteux F, Borderie D, Chapron C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod., 30(1), 49–60 (2015) https://doi.org/10.1093/humrep/deu290
Song Y, Liu J, Qiu Z, Chen D, Luo C, Liu X, Hua R, Zhu X, Lin Y, Li L, Liu W, Quan S. Advanced oxidation protein products from the follicular microenvironment and their role in infertile women with endometriosis. Exp. Ther. Med., 13(5), 2217–2222 (2017) https://doi.org/10.3892/etm.2017.5390
Ribeiro JC, Braga PC, Martins AD, Silva BM, Alves MG, Oliveira PF. Antioxidants present in reproductive tract fluids and their relevance for fertility. Antioxidants, 10(9), 1441 (2021) https://doi.org/10.3390/antiox10091441
Combelles CMH, Holick EA, Paolella LJ, Walker DC, Wu Q. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction, 139(5), 871–881 (2010) https://doi.org/10.1530/REP-09-0390
Yalcinkaya E, Cakiroglu Y, Doger E, Budak O, Cekmen M, Caliskan E. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J. Turk. Ger. Gynecol. Assoc., 14(3), 136–141 (2013) https://doi.org/10.5152/jtgga.2013.53323
Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil. Steril., 89(4), 912–921 (2008) https://doi.org/10.1016/j.fertnstert.2007.04.034.
Reiter RJ, Tan DX, Galano A. Melatonin: Exceeding expectations. Physiology (Bethesda), 29(5), 325–333 (2014). https://doi.org/10.1152/physiol.00011.2014
Hu L, Ye H, Wang S, Zhang Y. Melatonin application in assisted reproductive technology: a systematic review and meta-analysis. Front. Endocrinol., 11, 160 (2020) https://doi.org/10.3389/fendo.2020.00160
Tian X, Wang F, Zhang L, Ji P, Wang J, Lv D, Li G, Chai M, Lian Z, Liu G. Melatonin promotes the in vitro development of microinjected pronuclear mouse embryos via its anti-oxidative and anti-apoptotic effects. Int. J. Mol. Sci., 18(5), 988 (2017) https://doi.org/10.3390/ijms18050988
Ezzati M, Roshangar L, Rad JS, Karimian N. Evaluating the effect of melatonin on HAS2, and PGR expression, as well as cumulus expansion, and fertility potential in mice. Cell J. (Yakhteh), 20(1), 108 (2018). https://doi.org/10.22074/cellj.2018.4894
Keshavarzi S, Salehi M, Farifteh-Nobijari F, Hosseini T, Hosseini S, Ghazifard A, Ghaffari Novin M, Fallah-Omrani V, Nourozian M, Hosseini A. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell J. (Yakhteh), 20(2), 244 (2018) https://doi.org/10.22074/cellj.2018.4860
Jia Y, Liu W, Bai D, Zhang Y, Li Y, Liu Y, Yin J, Chen Q, Ye M, Zhao Y, Kou X, Wang H, Gao S, Li K, Chen M. Melatonin supplementation in the culture medium rescues impaired glucose metabolism in IVF mice offspring. J. Pineal Res., 72(1) (2022) https://doi.org/10.1111/jpi.12778
Kang J, Koo O, Kwon D, Park H, Jang G, Kang S, Lee B. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res., 46(1), 22–28 (2009) https://doi.org/10.1111/j.1600-079X.2008.00602.x
Martinez CA, Cuello C, Parrilla I, Maside C, Ramis G, Cambra JM, Vazquez JM, Rodriguez-Martinez H, Gil MA, Martinez EA. Exogenous melatonin in the culture medium does not affect the development of in vivo-derived pig embryos but substantially improves the quality of in vitro-produced embryos. Antioxidants, 11(6), 1177 (2022) https://doi.org/10.3390/antiox11061177
Lee S, Jin JX, Taweechaipaisankul A, Kim GA, Lee BC. Stimulatory effects of melatonin on porcine in vitro maturation are mediated by MT2 receptor. Int. J. Mol. Sci., 19(6), 1581 (2018) https://doi.org/10.3390/ijms19061581
Tian X, Wang F, Zhang L, He C, Ji P, Wang J, Zhang Z, Lv D, Abulizi W, Wang X, Lian Z, Liu G. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci., 18(4), 834 (2017) https://doi.org/10.3390/ijms18040834
Marques T, da Silva Santos E, Diesel T, Leme L, Martins C, Dode M, Alves B, Costa F, de Oliveira E, Gambarini M. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim., 53(1), 226–236 (2018) https://doi.org/10.1111/rda.13097
Zhao X, Wang N, Hao H, Li C, Zhao Y, Yan C, Wang H, Du W, Wang D, Liu Y, Pang Y, Zhu H. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res., 64(1) (2018) https://doi.org/10.1111/jpi.12445
Liu MJ, Sun AG, Zhao SG, Liu H, Ma SY, Li M, Huai YX, Zhao H, Liu HB. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril., 109(5), 900–907 (2018) https://doi.org/10.1016/j.fertnstert.2018.01.020
Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology, 83(8), 1360–1367 (2015) https://doi.org/10.1016/j.theriogenology.2015.01.029
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Chi MM, Xu J, Moley KH, Hennebold JD. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell, 14(5), 887–895 (2015) https://doi.org/10.1111/acel.12368
Wang F, Tian X, Zhang L, He C, Ji P, Li Y, Tan D, Liu G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril., 101(2), 577–586 (2014) https://doi.org/10.1016/j.fertnstert.2013.10.041
Luddi A, Capaldo A, Focarelli R, Gori M, Morgante G, Piomboni P, de Leo V. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod. Biol. Endocrinol., 14(1), 57 (2016) https://doi.org/10.1186/s12958-016-0184-7
Sadeghi R, Shokrzadeh M, Hashemi M, Jafarzadeh A. Effect of coenzyme Q10 supplementation on oocyte and embryo quality in older women undergoing IVF: A randomized controlled trial. Reprod. Biol., 23(1), 56–64 (2023) https://doi.org/10.1016/j.repbio.2022.11.003
Dahri M, Sadeghi AS, Pahlavani N, Nattagh-Eshtivani E, Hashemilar M, Asghari-Jafarabadi M, Tarighat-Esfanjani A. The effects of coenzyme Q10 supplementation on oxidative status and lipid profile in migraine patients: A randomized double-blinded controlled clinical trial. Clin. Nutr. Res., 12(4), 257 (2023). https://doi.org/10.7762/cnr.2023.12.4.257
Gairola N, Chitme HR, Sircar R. Systemic Effect of Human Follicular Fluid from Endometriotic and Healthy Subjects on Female Mice. Indian J. Pharm. Educ. Res., 58(2s), s660–s667 (2024) https://doi.org/10.5530/ijper.58.2s.70.
Zhang Y, Li L, Wang H, Xu M. Influence of antioxidant duration on reproductive outcomes in IVF patients: Balancing efficacy and safety. Reprod. Biol., 23(2), 102–110 (2023) https://doi.org/10.1016/j.repbio.2023.03.005
Published
How to Cite
Issue
Section
Copyright (c) 2025 Nidhi Gairola, Himanko Gogoi, Janhvi Dubey, Jasvinder Singh Khatiyaan, Harsh Chaudhary

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















