Chitosan-based in-situ forming polyelectrolyte complexes for ciprofloxacin sustained release tablets
DOI:
https://doi.org/10.69857/joapr.v13i3.1034Keywords:
Ciprofloxacin hydrochloride, Chitosan, Polyelectrolyte complex, Sodium Starch Glycolate, Xanthan GumAbstract
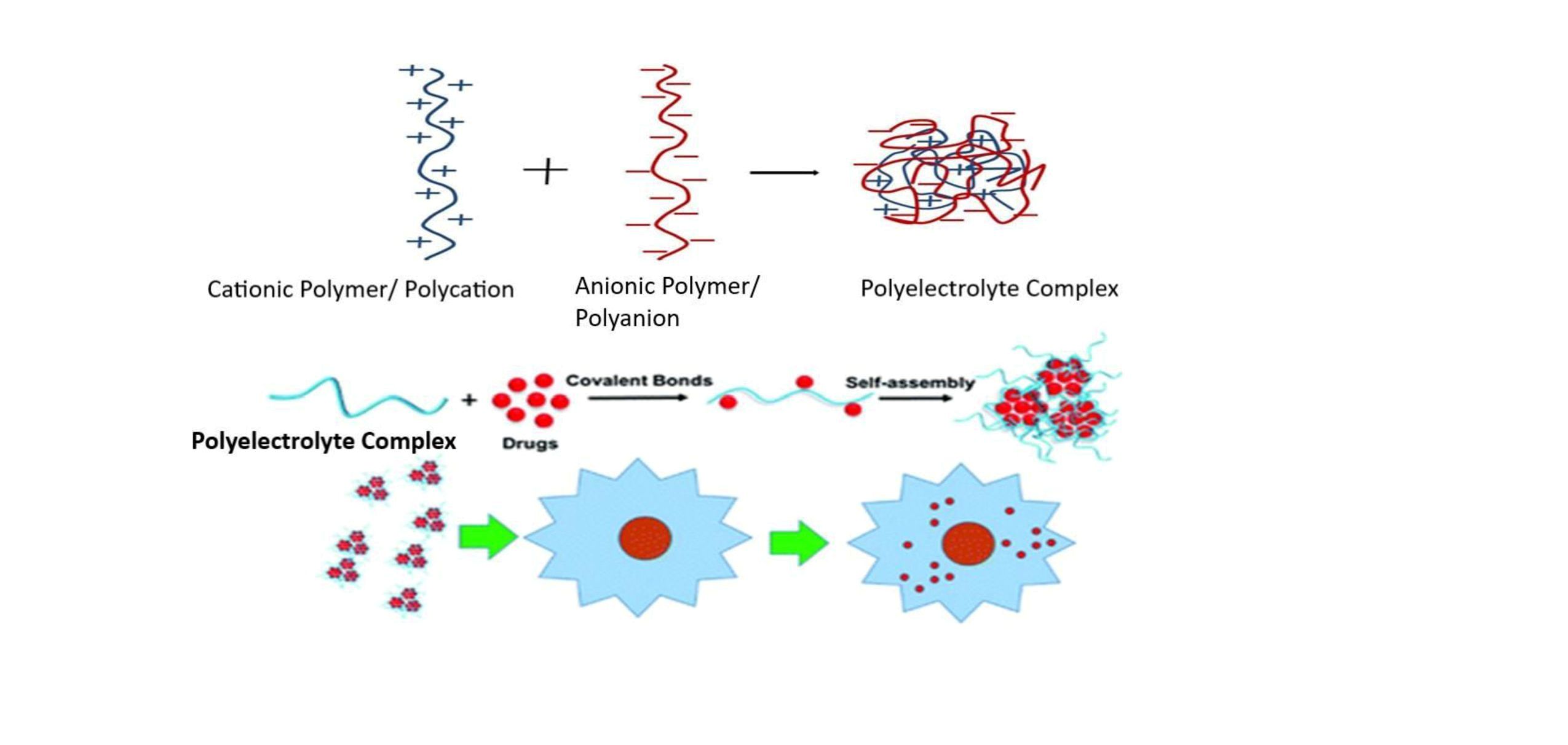
Background: It is not straightforward to create sustained-release (single-unit) oral dosage forms for hydrophilic medicines, which are highly soluble (10 mg/mL) in gastric fluids and have a high dose. This study is an attempt to utilize biopolymer Chitosan-based Polyelectrolyte complex as a retardant to develop and evaluate the sustained release tablet formulations (oral) of Ciprofloxacin hydrochloride. Methodology: Sustained-release tablets were prepared using the traditional wet granulation method, employing a neutralized chitosan solution (1% w/w) at 4°C in 1% acetic acid as the binder. Formulated tablets were assessed for pharmacopoeial and non-pharmacopoeial parameters, as well as in vitro 12-hour drug release studies. The different mathematical models were utilized to examine the pharmacokinetic parameters and elucidate the mechanism of drug release. Results and discussion: The sustained release of the drug for 12 hrs was confirmed through the in vitro release studies. Both formulations, CFX 2 and CFX 3 exhibited 97% and 98% cumulative drug release, respectively, after 12 hr. The dissolution profiles of both formulations were shown to be unaffected by the change in anionic polymers from one to two, as confirmed by dissolution profile comparison studies, with values of similarity factor (f2) 84 and of difference factor (f1) 2. The XRD studies confirmed the in situ formation of a polyelectrolyte complex between chitosan and anionic polymer, as evidenced by the presence of additional peaks in the diffractograms. Conclusion: The polyelectrolyte complexes not only provide a sustained drug release but also prevent the initial burst release of the the drug.
Downloads
References
Mahmoud ZH, Hamrouni A, Kareem AB, Mostafa MA, Jalil alhakim Z, Majeed AH. Synthesis and characterization of chitosan sheet modified by varied weight ratio of anatase (TiO2) nano mixture with Cr(VI) adsorbing. Kuwait J. Sci., 50, 290–9 (2023) https://doi.org/10.1016/j.kjs.2023.05.006.
Lal N, Dubey J, Gaur P, Verma N, Verma A. Chitosan based in situ forming polyelectrolyte complexes: A potential sustained drug delivery polymeric carrier for high dose drugs. Mater. Sci. Eng. C. Mater. Biol. Appl., 79, 491–8 (2017) https://doi.org/10.1016/j.msec.2017.05.051.
Verma A, Bansal A, Ghosh A, Pandit J. Low molecular mass chitosan as carrier for a hydrodynamically balanced system for sustained delivery of ciprofloxacin hydrochloride. Acta Pharm., 62, 237–50 (2012) https://doi.org/10.2478/v10007-012-0013-2.
Moed S, Hall M, Lee N, Costa C P, Rodland E K, Shemirani A I, Clifford K, Desai D, Zaman M H, Quantitative assay for ciprofloxacin and enrofloxacin formulations. JoGR,3, e2019044 (2019) https://doi.org/10.29392/joghr.3.e2019044
Mahmoud TY, Hamza IS, Jarallah AL. Spectrophotometric Method for the Determination of Ciprofloxacin in Pure and Pharmaceutical Preparations: Development and Validation. Engineering Proceedings, 59(1), 164 (2023) https://doi.org/10.3390/engproc2023059164
Al-Omar M. Ciprofloxacin: Analytical Profile. Profiles Drug Subst. Excip. Relat. Methodol., 31, 179–207 (2005) https://doi.org/10.1016/S0099-5428(04)31005-1.
Prasad AR, Ratna JV. Development and validation of a simple uv-spectrophotometric method for the determination of ciprofloxacin HCl present in taste masked drug resin complex. Int. J. Appl. Pharm., 10, 37–41 (2018) https://doi.org/10.22159/ijap.2018v10i3.24199.
Sahu A, Chouksey K, Ganju K. Formulation and Evaluation of Ciprofloxacin Hydrochloride Sustained Release Tablets Using Hibiscus Rosa Sinensis Mucilage. J. Adv. Sci. Res., 13, 71–8 (2022) https://doi.org/10.55218/jasr.202213811
Kahsu A, Aklilu T, Masresh B, Melkam W. Benefits and Risks of Fluoroquinolones Use in Pediatrics: a Review. Int. J. Life Sci. Rev. Rev. Artic. Int. J. Life Sci. Rev., 1, 169–74 (2015) https://doi.org/10.13040/IJPSR.0975-8232.IJLSR.1.
Mocanu AG, Belu I, Croitoru O, Ciocîlteu MV, Manda CV, Neamtu J. Formulation and Characterization of Ciprofloxacin loaded PLGA Microspheres for Applications in Orthopedic Infections. Curr Health Sci J., 43(4), 306-310 (2017) https://doi.org/10.12865/CHSJ.43.04.03.
Fahmy S, Abu-Gharbieh E. In vitro dissolution and in vivo bioavailability of six brands of ciprofloxacin tablets administered in rabbits and their pharmacokinetic modeling. Biomed Res Int., 2014, 590848 (2014) https://doi.org/10.1155/2014/590848.
J. Conceicao, M. Estanqueiro, M. H. Amaral, P. Lobao, P. Costa, J. M. Sousa Lobo. Development and Characterization of Buccal Bilayer Tablets containing Microparticles with Ibuprofen. Am. J. Med. Sci. Med.; 2(5):109-114. (2014) https://doi.org/10.12691/ajmsm-2-5-5.
Shah VP, Tsong Y, Sathe P, Liu J-P. In Vitro Dissolution Profile Comparison—Statistics and Analysis of the Similarity Factor, f2. Pharm. Res., 15, 889–96 (1998) https://doi.org/10.1023/A:1011976615750.
Skelly JP, Amidon GL, Barr WH, Benet LZ, Carter JE, Robinson JR, Shah VP, Yacobi A. In Vitro and in Vivo Testing and Correlation for Oral Controlled/Modified-Release Dosage Forms. Pharm. Res., 7, 975–82 (1990) https://doi.org/10.1023/A:1015970512368.
Samaha D, Shehayeb R, Kyriacos S. Modeling and comparison of dissolution profiles of diltiazem modified-release formulations. Dissolution Technol., 16, 41–6 (2009) https://doi.org/10.14227/DT160209P41.
Tom RT, Suryanarayanan V, Reddy PG, Baskaran S, Pradeep T. Ciprofloxacin-protected gold nanoparticles. Langmuir, 20, 1909–14 (2004) https://doi.org/10.1021/la0358567.
Leonida M, Ispas-Szabo P, Mateescu MA. Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery. Bioact. Mater., 3, 334–40 (2018) https://doi.org/10.1016/j.bioactmat.2018.04.001.
Shah KA, Li G, Song L, Gao B, Huang L, Luan D, Iqbal H, Cao Q, Menaa F, Lee B-J, Alnasser, S. M., Alshahrani, S. M., & Cui, J. Rizatriptan-Loaded Oral Fast Dissolving Films: Design and Characterizations. Pharmaceutics. 14 (12), 2687 (2022) https://doi.org/10.3390/pharmaceutics14122687
Jadhav RL, Beloshe P, Siraj S, Vyankatrao PM. Design, Development, and Characterization of Modified Xanthan Gum Based Novel in situ Gel of Ciprofloxacin Hydrochloride for Ophthalmic Drug Delivery. Asian J. Pharm., 14, 236–46 (2020) https://doi.org/10.22377/ajp.v14i2.3619.
Lazaridou A, Biliaderis C G. Thermophysical properties of chitosan, chitosan–starch and chitosan–pullulan films near the glass transition Carbohydrate Polymers, 48 (2), 179-190 (2002) https://doi.org/10.1016/S0144-8617(01)00261-2
Dong Y, Ruan Y, Wang H, Zhao Y, Bi D. Studies on glass transition temperature of chitosan with four techniques. J. Appl. Polym. Sci., 93, 1553–8 (2004) https://doi.org/10.1002/app.20630.
Zaid H. Mahmoud, AchrafHamrouni, Asmaa B. Kareem, Mohammed Ahmed Mostafa, ZaharaJalilalhakim, Abdulwahhab H. Majeed. Synthesis and characterization of chitosan sheet modified by varied weight ratio of anatase (TiO2) nano mixture with Cr(VI) adsorbing. Kuwait Journal of Science, 50 (3), 290-299 (2023) https://doi.org/10.1016/j.kjs.2023.05.006.
Kumar S, Koh J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci., 13, 6102–16 (2012) https://doi.org/10.3390/ijms13056102.
Bhandari PN, Jones DD, Hanna MA. Characterization of sodium starch glycolate prepared using reactive extrusion and its comparisons with a commercial brand VIVASTAR ®P. Ind. Crops Prod., 41, 324–30 (2013) https://doi.org/10.1016/j.indcrop.2012.04.050.
Chaulang G, Patel P, Hardikar S, Kelkar M, Bhosale A, Bhise S. Formulation and evaluation of solid dispersions of furosemide in sodium starch glycolate. Trop. J. Pharm. Res., 8, 43–51 (2009) https://doi.org/10.4314/tjpr.v8i1.14711.
Kashaudhan K, Pande PP, Sharma J, Shankar R, Nath A, Chaurasiya A, Kushwaha N. Synthesis and characterization of Xanthan Gum Xanthates and their application for toxic metal ion removal from synthetic wastewater. J. Dispers. Sci. Technol., 0, 1–15 (2024) https://doi.org/10.1080/01932691.2024.2373932.
Elena VAizquez, SofAa Piguillem, Santiago Rubio, Jorge DAaz, Hector Baldoni EV and MM. Structural Analysis of Xanthan GUM-FE (III) Capsules. Acad. J. Chem., 5, 31–40 (2020) https://doi.org/10.32861/ajc.54.31.40.
Zheng M, Lian F, Xiong Y, Liu B, Zhu Y, Miao S, Zhang L, Zheng B. The synthesis and characterization of a xanthan gum-acrylamide-trimethylolpropane triglycidyl ether hydrogel. Food Chem., 272, 574–9 (2019) https://doi.org/10.1016/j.foodchem.2018.08.083.
Sahoo S, Chakraborti CK, Naik S, Mishra SC, Nanda UN. Structural analysis of ciprofloxacin-carbopol polymeric composites by X-ray diffraction and fourier transform infra-red spectroscopy. Trop. J. Pharm. Res., 10, 273–80 (2011) https://doi.org/10.4314/tjpr.v10i3.14.
Lee BJ, Ryu SG, Cui JH. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev. Ind. Pharm., 25, 493–501 (1999) https://doi.org/10.1081/ddc-100102199.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Ajay Malik, Navneet Verma, Vijay Sharma

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















