Overcoming chemoresistance in mucinous adenocarcinoma: the impact of tumor microenvironment and genetic alterations
DOI:
https://doi.org/10.69857/joapr.v13i3.1032Keywords:
Mucinous adenocarcinoma, Chemoresistance, Targeted therapy, Tumor microenvironment, Cancer stem cells, NanomedicineAbstract
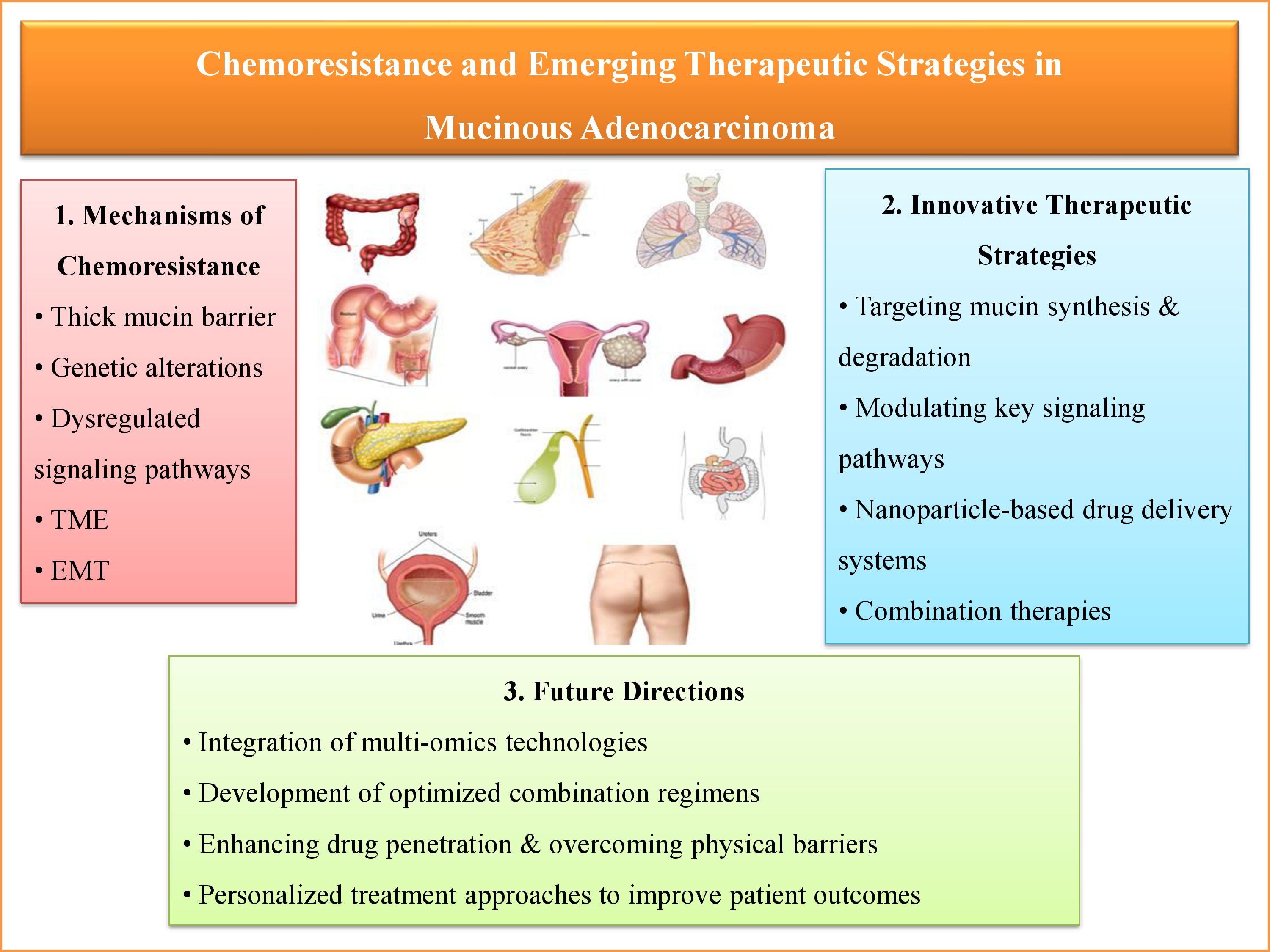
Background: Mucinous adenocarcinoma (MAC) is a rare, aggressive subtype of adenocarcinoma, distinguished by excessive extracellular mucin production. This feature impairs drug penetration and contributes to poor chemotherapy response and chemoresistance. Genetic mutations (e.g., KRAS, BRAF, PI3K/AKT), epithelial-to-mesenchymal transition (EMT), alterations in the tumor microenvironment, and mucin barriers contribute to this resistance. Objective: This narrative review aims to comprehensively summarize the molecular and microenvironmental mechanisms behind chemoresistance in MAC and highlight emerging therapeutic strategies to overcome it. Results: Chemoresistance in MAC arises from oncogenic signaling, immune evasion, hypoxia, and mucin-mediated drug exclusion. Promising approaches include mucolytic agents, small-molecule inhibitors, immune checkpoint inhibitors, RNA-based therapies, and nanoparticle-assisted drug delivery. Precision medicine, which utilizes genomic and transcriptomic profiling, is advancing individualized treatment; however, clinical translation remains limited. Conclusion: Resistance in MAC stems from both genetic and microenvironmental factors. Understanding these mechanisms is crucial for developing more effective, personalized therapies to improve patient outcomes. Future efforts should focus on validating novel therapies through clinical trials, discovering predictive biomarkers, and exploring tumor heterogeneity with multi-omics technologies. Integrating targeted therapies with advanced delivery systems may offer significant advances in treating chemoresistant MAC.
Downloads
References
Sekaran S, Warrier S, Selvaraj V, Ganapathy D, Ramasamy P. NLRP3 Inflammasome: A Potential Therapeutic Target in Head and Neck Cancers. Clinical oncology (Royal College of Radiologists (Great Britain)), 36(5), e115–e117 (2024) https://doi.org/10.1016/j.clon.2024.02.007.
Veeraraghavan VP, Mony U, Renu K, Surapaneni KM, Ammar RB, AlZahrani AM, Ahmed EA, Rajendran P. Effects of polyphenols on ncRNAs in cancer-An update. Clinical and experimental pharmacology & physiology, 49(6), 613–623 (2022) https://doi.org/10.1111/1440-1681.13641.
Vasudevan S, Kannan K, Raghavan AV, Sulochana S. Analyzing Tumor Budding Scores in Invasive Breast Carcinoma: A Tertiary Care Center Study in South India. Journal of pharmacy & bioallied sciences, 16(2), S1850–S1853 (2024) https://doi.org/10.4103/jpbs.jpbs_910_23.
Breugelmans T, Oosterlinck B, Arras W, Ceuleers HDe Man J, Hold GL, De Winter BY, Smet A. The role of mucins in gastrointestinal barrier function during health and disease. The lancet. Gastroenterology & hepatology, 7(5), 455–471 (2022) https://doi.org/10.1016/S2468-1253(21)00431-3.
Gautam S K, Khan P, Natarajan G, Atri P, Aithal A, Ganti A K, Batra S K, Nasser M W, Jain M. Mucins as Potential Biomarkers for Early Detection of Cancer. Cancers, 15(6), 1640 (2023) https://doi.org/10.3390/cancers15061640.
Wi DH, Cha JH, Jung YS. Mucin in cancer: a stealth cloak for cancer cells. BMB reports, 54(7), 344–355 (2021) https://doi.org/10.5483/BMBRep.2021.54.7.064.
Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Archives of pathology & laboratory medicine, 145(6), 664–677 (2021) https://doi.org/10.5858/arpa.2019-0665-RA.
Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID. Insight into mucinous colorectal carcinoma: clues from etiology. Annals of surgical oncology, 21(9), 2963–2970 (2014) https://doi.org/10.1245/s10434-014-3706-6.
van der Meer DJ, Kramer I, van Maaren MC, van Diest PJ C Linn S, Maduro JH, J A Strobbe L, Siesling S, Schmidt MK, Voogd AC. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. International journal of cancer, 148(9), 2289–2303 (2021) https://doi.org/10.1002/ijc.33417.
De Abreu Pereira D, Areias VR, Franco MF, Benitez MC, do Nascimento CM, de Azevedo CM, Alves G. Measurement of HER2 in saliva of women in risk of breast cancer. Pathology oncology research : POR, 19(3), 509–513 (2013) https://doi.org/10.1007/s12253-013-9610-8.
Ragavendran C. Letter to the editor regarding, "Development and validation of a relatively accurate gastric cancer high-risk group screening scoring system in urban residents". Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 10.1007/s12094-024-03806-Advance online publication (2024) https://doi.org/10.1007/s12094-024-03806-9.
Kodipalli A, Fernandes SL, Dasar S. An Empirical Evaluation of a Novel Ensemble Deep Neural Network Model and Explainable AI for Accurate Segmentation and Classification of Ovarian Tumors Using CT Images. Diagnostics (Basel, Switzerland), 14(5), 543 (2024) https://doi.org/10.3390/diagnostics14050543.
Srinivasan S, Mohanprasanth A, Nadeem A, Saravanan M. Exploring the anti-cancer and antimetastatic effect of Silymarin against lung cancer. Toxicology reports, 13, 101746 (2024) https://doi.org/10.1016/j.toxrep.2024.101746.
Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World journal of gastrointestinal surgery, 13(12), 1567–1583 (2021) https://doi.org/10.4240/wjgs.v13.i12.1567.
Cai G, Xu Y, Lu H, Shi Y, Lian P, Peng J, Du X, Zhou X, Guan Z, Shi D, Cai S. Clinicopathologic and molecular features of sporadic microsatellite- and chromosomal-stable colorectal cancers. International journal of colorectal disease, 23(4), 365–373 (2008) https://doi.org/10.1007/s00384-007-0423-7.
Soliman BG, Karagkounis G, Church JM, Plesec T, Kalady MF. Mucinous Histology Signifies Poor Oncologic Outcome in Young Patients With Colorectal Cancer. Diseases of the colon and rectum, 61(5), 547–553 (2018) https://doi.org/10.1097/DCR.0000000000001060.
Rosberg V, Jessen M, Qvortrup C, Smith HG, Krarup PM. Impact of adjuvant chemotherapy on long-term overall survival in patients with high-risk stage II colon cancer: a nationwide cohort study. Acta oncologica (Stockholm, Sweden), 62(9), 1076–1082 (2023) https://doi.org/10.1080/0284186X.2023.2251086.
Hogan J, Burke JP, Samaha G, Condon E, Waldron D, Faul P, Coffey JC. Overall survival is improved in mucinous adenocarcinoma of the colon. International journal of colorectal disease, 29(5), 563–569 (2014) https://doi.org/10.1007/s00384-013-1826-2.
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. Journal of breast cancer, 14(4), 308–313 (2011) https://doi.org/10.4048/jbc.2011.14.4.308.
Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, Wang F, Li S. Clinical Nomogram for Predicting Survival Outcomes in Early Mucinous Breast Cancer. PloS one, 11(10), e0164921 (2016) https://doi.org/10.1371/journal.pone.0164921.
Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, Shepherd JH, Ind, T, Bridges, J, Harrington K, Kaye SB, Gore ME. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 22(6), 1040–1044 (2004) https://doi.org/10.1200/JCO.2004.08.078.
Craig O, Salazar C, Gorringe KL. Options for the Treatment of Mucinous Ovarian Carcinoma. Current treatment options in oncology, 22(12), 114 (2021) https://doi.org/10.1007/s11864-021-00904-6.
Shoji T, Tatsuki S, Abe M, Tomabechi H, Takatori E, Kaido Y, Nagasawa T, Kagabu M, Baba T, Itamochi H. Novel Therapeutic Strategies for Refractory Ovarian Cancers: Clear Cell and Mucinous Carcinomas. Cancers, 13(23), 6120 (2021) https://doi.org/10.3390/cancers13236120.
Craig O, Salazar C, Gorringe KL. Options for the Treatment of Mucinous Ovarian Carcinoma. Current treatment options in oncology, 22(12), 114 (2021) https://doi.org/10.1007/s11864-021-00904-6.
Deppe G, Baumann P. Advances in ovarian cancer chemotherapy. Current opinion in oncology, 12(5), 481–491 (2000) https://doi.org/10.1097/00001622-200009000-00016.
Wagner CE, Wheeler KM, Ribbeck K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annual review of cell and developmental biology, 34, 189–215 (2018) https://doi.org/10.1146/annurev-cellbio-100617-062818.
Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nature reviews. Gastroenterology & hepatology, 10(10), 607–620 (2013) https://doi.org/10.1038/nrgastro2013.120.
Lin WH, Cooper LM, Anastasiadis PZ. Cadherins and catenins in cancer: connecting cancer pathways and tumor microenvironment. Frontiers in cell and developmental biology, 11, 1137013 (2023) https://doi.org/10.3389/fcell.2023.1137013.
Jonckheere N, Skrypek N, Van Seuningen, I. Mucins and tumor resistance to chemotherapeutic drugs. Biochimica et biophysica acta, 1846(1), 142–151 (2014) https://doi.org/10.1016/j.bbcan.2014.04.008.
Ueda S, Saeki T, Osaki A, Yamane T, Kuji I. Bevacizumab Induces Acute Hypoxia and Cancer Progression in Patients with Refractory Breast Cancer: Multimodal Functional Imaging and Multiplex Cytokine Analysis. Clinical cancer research : an official journal of the American Association for Cancer Research, 23(19), 5769–5778 (2017) https://doi.org/10.1158/1078-0432.CCR-17-0874.
Alabiad M., Harb O, Mandour D, Hemeda R, Z Ahmed R, El-Taher A, Osman G, Shalaby A, A A Alnemr A, T Abdelfattah M. Prognostic and clinicopathological implications of expression of Beclin-1 and hypoxia-inducible factor 1α in serous ovarian carcinoma: an immunohistochemical study. Polish journal of pathology : official journal of the Polish Society of Pathologists, 72(1), 23–38 (2021) https://doi.org/10.5114/pjp.2021.106441.
Martínez-Aguilar R, Kershaw LE, Reavey JJ, Critchley HOD, Maybin JA. HYPOXIA AND REPRODUCTIVE HEALTH: The presence and role of hypoxia in the endometrium. Reproduction (Cambridge, England), 161(1), F1–F17 (2021) https://doi.org/10.1530/REP-20-0268.
Yin H, Pu N, Chen Q, Zhang J, Zhao G, Xu X, Wang D, Kuang T, Jin D, Lou W, Wu W. Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-κB pathway in pancreatic cancer. Cell death & disease, 12(11), 1033 (2021) https://doi.org/10.1038/s41419-021-04293-4.
Zhitomirsky B, Assaraf YG. Lysosomes as mediators of drug resistance in cancer. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy, 24, 23–33 (2016) https://doi.org/10.1016/j.drup.2015.11.004.
Zub K A, Sousa MM, Sarno A, Sharma A, Demirovic A, Rao S, Young C, Aas PA, Ericsson I, Sundan A, Jensen ON, Slupphaug G. Modulation of cell metabolic pathways and oxidative stress signaling contribute to acquired melphalan resistance in multiple myeloma cells. PloS one, 10(3), e0119857 (2015) https://doi.org/10.1371/journal.pone.0119857.
Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. The Journal of clinical investigation, 126(10), 3689–3698 (2016) https://doi.org/10.1172/JCI84430.
Silva VL, Al-Jamal WT. Exploiting the cancer niche: Tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. Journal of controlled release : official journal of the Controlled Release Society, 253, 82–96 (2017) https://doi.org/10.1016/j.jconrel.2017.03.013.
Horiuchi M, Uemura T, Oguri T, Toda S, Yamamoto S, Suzuki Y, Kagawa Y, Sone K, Fukuda S, Mori Y, Fukumitsu K, Kanemitsu Y, Tajiri T, Ohkubo H, Takemura M, Ito Y, Maeno K, Niimi A. Genetic variations in the ATP-binding cassette transporter ABCC10 are associated with neutropenia in Japanese patients with lung cancer treated with nanoparticle albumin-bound paclitaxel. Investigational new drugs, 40(5), 934–943 (2022) https://doi.org/10.1007/s10637-022-01275-x.
Sunami Y, Böker V, Kleeff J. Targeting and Reprograming Cancer-Associated Fibroblasts and the Tumor Microenvironment in Pancreatic Cancer. Cancers, 13(4), 697 (2021) https://doi.org/10.3390/cancers13040697.
Sperb N, Tsesmelis M, Wirth T. Crosstalk between Tumor and Stromal Cells in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci, 21(15), 5486 (2020) https://doi.org/10.3390/ijms21155486
Harryvan TJ, Golo M, Dam N, Schoonderwoerd MJA, Farshadi EA, Hornsveld M, Hoeben RC, Hawinkels LJAC, Kemp V. Gastrointestinal cancer-associated fibroblasts expressing Junctional Adhesion Molecule-A are amenable to infection by oncolytic reovirus. Cancer gene therapy, 29(12), 1918–1929 (2022) https://doi.org/10.1038/s41417-022-00507-9
Greco L, Rubbino F, Laghi L. Epithelial to Mesenchymal Transition as Mechanism of Progression of Pancreatic Cancer: From Mice to Men. Cancers, 14(23), 5797 (2022) https://doi.org/10.3390/cancers14235797
Sarkar R, Xu Z, Perera C. J, Apte M V. Emerging role of pancreatic stellate cell-derived extracellular vesicles in pancreatic cancer. Seminars in cancer biology, 93, 114–(2023) https://doi.org/10.1016/j.semcancer.2023.05.007.
Liu Y, Su Z, Tavana O, Gu W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer cell, 42(6), 946–967 (2024) https://doi.org/10.1016/j.ccell.2024.04.009.
Dearden S, Stevens J, Wu Y L, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Annals of oncology : official journal of the European Society for Medical Oncology, 24(9), 2371–2376 (2013) https://doi.org/10.1093/annonc/mdt205
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature, 511(7511), 543–550 (2014) https://doi.org/10.1038/nature13385.
Shajani-Yi Z, de Abreu F B, Peterson J D, Tsongalis G J. Frequency of Somatic TP53 Mutations in Combination with Known Pathogenic Mutations in Colon Adenocarcinoma, Non-Small Cell Lung Carcinoma, and Gliomas as Identified by Next-Generation Sequencing. Neoplasia (New York, N.Y.), 20(3), 256–262 (2018) https://doi.org/10.1016/j.neo.2017.12.005.
Gabriel A A G, Atkins J R, Penha R C C, Smith-Byrne K, Gaborieau V, Voegele C, Abedi-Ardekani B, Milojevic M, Olaso R, Meyer V, Boland A, Deleuze J F, Zaridze D, Mukeriya A, Swiatkowska B, Janout V, Schejbalová M, Mates D, Stojšić J, Ognjanovic M, McKay J D. Genetic Analysis of Lung Cancer and the Germline Impact on Somatic Mutation Burden. Journal of the National Cancer Institute, 114(8), 1159–1166 (2022) https://doi.org/10.1093/jnci/djac087.
Sanmamed M F, Chen LA Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell, 175(2), 313–326 (2018) https://doi.org/10.1016/j.cell.2018.09.035.
Skoulidis F, Goldberg M E, Greenawalt D M, Hellmann M D, Awad M M, Gainor J F, Schrock A B, Hartmaier R J, Trabucco S E, Gay L, Ali S M, Elvin J A, Singal G, Ross J S, Fabrizio D, Szabo P M, Chang H, Sasson A, Srinivasan S, Kirov S, Heymach J V. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer discovery, 8(7), 822–835 (2018) https://doi.org/10.1158/2159-8290.CD-18-0099.
McCleary-Wheeler A L, Lomberk G A, Weiss F U, Schneider G, Fabbri M, Poshusta T L, Dusetti N J, Baumgart S, Iovanna J L, Ellenrieder V, Urrutia R, Fernandez-Zapico M E. Insights into the epigenetic mechanisms controlling pancreatic carcinogenesis. Cancer letters, 328(2), 212–221 (2013) https://doi.org/10.1016/j.canlet.2012.10.005.
Angeloni A, Bogdanovic O. Sequence determinants, function, and evolution of CpG islands. Biochemical Society transactions, 49(3), 1109–1119 (2021) https://doi.org/10.1042/BST20200695.
Zhou X, Hu K, Bailey P, Springfeld C, Roth S, Kurilov R, Brors B, Gress T, Buchholz M, An J, Wei K, Peccerella T, Büchler M W, Hackert T, Neoptolemos J P. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Frontiers in cell and developmental biology, 9, 743908 (2021) https://doi.org/10.3389/fcell.2021.743908.
Lomberk G A, Iovanna J, Urrutia R. The promise of epigenomic therapeutics in pancreatic cancer. Epigenomics, 8(6), 831–842 (2016) https://doi.org/10.2217/epi-2015-0016.
Hessmann E, Johnsen SA, Siveke JT, Ellenrieder V. Epigenetic treatment of pancreatic cancer: is there a therapeutic perspective on the horizon?. Gut, 66(1), 168–179 (2017) https://doi.org/10.1136/gutjnl-2016-312539.
Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Current medicinal chemistry, 24(13), 1321–1331 (2017) https://doi.org/10.2174/0929867324666170206142658.
Chen S, Chen C, Hu Y, Song G, Shen X. The diverse roles of circular RNAs in pancreatic cancer. Pharmacology & therapeutics, 226, 107869 (2021) https://doi.org/10.1016/j.pharmthera.2021.107869.
Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cellular and molecular life sciences : CMLS, 72(12), 2337–2347 (2015) https://doi.org/10.1007/s00018-015-1867-5.
Degan SE, Gelman IH. Emerging Roles for AKT Isoform Preference in Cancer Progression Pathways. Molecular cancer research : MCR, 19(8), 1251–1257 (2021) https://doi.org/10.1158/1541-7786.MCR-20-1066.
Baer R, Cintas C, Therville N, Guillermet-Guibert J. Implication of PI3K/Akt pathway in pancreatic cancer: When PI3K isoforms matter?. Advances in biological regulation, 59, 19–35 (2015) https://doi.org/10.1016/j.jbior.2015.05.001.
Mehra S, Deshpande N, Nagathihalli N. Targeting PI3K Pathway in Pancreatic Ductal Adenocarcinoma: Rationale and Progress. Cancers, 13(17), 4434 (2021) https://doi.org/10.3390/cancers13174434.
Wadhwa B, Makhdoomi U, Vishwakarma R, Malik F. Protein kinase B: emerging mechanisms of isoform-specific regulation of cellular signaling in cancer. Anti-cancer drugs, 28(6), 569–580 (2017) https://doi.org/10.1097/CAD.0000000000000496.
Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Seminars in cancer biology, 59, 147–160 (2019) https://doi.org/10.1016/j.semcancer.2019.05.012.
Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Seminars in cancer biology, 59, 125–132 (2019) https://doi.org/10.1016/j.semcancer.2019.07.009.
Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. European journal of medicinal chemistry, 109, 314–341 (2016) https://doi.org/10.1016/j.ejmech.2016.01.012.
Selvarajoo N, Stanslas J, Islam MK, Sagineedu SR, Lian HK, Lim JCW. Pharmacological Modulation of Apoptosis and Autophagy in Pancreatic Cancer Treatment. Mini reviews in medicinal chemistry, 22(20), 2581–2595 (2022) https://doi.org/10.2174/1389557522666220324123605.
Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, Sahraei M, Mukherjee P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis, 2(6), e51 (2013) https://doi.org/10.1038/oncsis.2013.16.
Shin DY. Human acute myeloid leukemia stem cells: evolution of concept. Blood research, 57(S1), 67–74 (2022) https://doi.org/10.5045/br.2022.2021221.
Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer research, 73(1), 8–11 (2013) https://doi.org/10.1158/0008-5472.CAN-12-3422.
Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, Huang L, Liu CC, Shao ZM, Yu KD. Breast cancer: pathogenesis and treatments. Signal transduction and targeted therapy, 10(1), 49 (2025) https://doi.org/10.1038/s41392-024-02108-4.
Loras A, Gonzalez-Bonet LG, Gutierrez-Arroyo JL, Martinez-Cadenas C, Marques-Torrejon M A. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life (Basel, Switzerland), 13(4), 905 (2023) https://doi.org/10.3390/life13040905.
Centeno PP, Pavet V, Marais R. The journey from melanocytes to melanoma. Nature reviews. Cancer, 23(6), 372–390 (2023) https://doi.org/10.1038/s41568-023-00565-7.
O'Sullivan É, Keogh A, Henderson B, Finn SP, Gray SG, Gately K. Treatment Strategies for KRAS-Mutated Non-Small-Cell Lung Cancer. Cancers, 15(6), 1635 (2023) https://doi.org/10.3390/cancers15061635.
Stanciu S, Ionita-Radu F, Stefani C, Miricescu D, Stanescu-Spinu II, Greabu M, Ripszky Totan A, Jinga M. Targeting PI3K/AKT/mTOR Signaling Pathway in Pancreatic Cancer: From Molecular to Clinical Aspects. International journal of molecular sciences, 23(17), 10132 (2022) https://doi.org/10.3390/ijms231710132.
Ghanaatgar-Kasbi S, Khazaei M, Rastgar-Moghadam A, Ferns GA, Hassanian SM, Avan A. The Therapeutic Potential of MEK1/2 Inhibitors in the Treatment of Gynecological Cancers: Rational Strategies and Recent Progress. Current cancer drug targets, 20(6), 417–428 (2020) https://doi.org/10.2174/1568009620666200424144303.
Thimmareddygari D, Ramahi A, Chan KH, Patel R, Bellary S, Sharma H, Miller R. An Unusual Presentation of Aggressive Primary Invasive Adenocarcinoma of Lung. The American journal of the medical sciences, 361(1), 118–125 (2021) https://doi.org/10.1016/j.amjms.2020.09.014.
Teer JK, Yoder S, Gjyshi A, Nicosia SV, Zhang C, Monteiro ANA. Mutational heterogeneity in non-serous ovarian cancers. Scientific reports, 7(1), 9728 (2017) https://doi.org/10.1038/s41598-017-10432-9.
Ricci F, Affatato R, Carrassa L, Damia G. Recent Insights into Mucinous Ovarian Carcinoma. International journal of molecular sciences, 19(6), 1569 (2018) https://doi.org/10.3390/ijms19061569.
Tafe LJ, Muller KE, Ananda G, Mitchell T, Spotlow V, Patterson SE, Tsongalis GJ, Mockus SM. Molecular Genetic Analysis of Ovarian Brenner Tumors and Associated Mucinous Epithelial Neoplasms: High Variant Concordance and Identification of Mutually Exclusive RAS Driver Mutations and MYC Amplification. The American journal of pathology, 186(3), 671–677 (2016) https://doi.org/10.1016/j.ajpath.2015.11.008.
Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, Halling K, Karnezis A, Senz J, Yang W, Prigge ES, Reuschenbach M, Doeberitz MV, Gilks BC, Huntsman DG, Bakkum-Gamez J, McAlpine JN, Anglesio MS. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC cancer, 15, 415 (2015) https://doi.org/10.1186/s12885-015-1421-8.
Florent L, Saby C, Slimano F, Morjani H. BRAF V600-Mutated Metastatic Melanoma and Targeted Therapy Resistance: An Update of the Current Knowledge. Cancers, 15(9), 2607 (2023) https://doi.org/10.3390/cancers15092607.
Park MK, Lee HJ, Sung JY, Byun HJ, Kim HJ, Kim EJ, Nguyen TM, Kang GJ, Oh SH, Shim JG, Lee H, Nam KT, Kim YY, Rho SB, Kim SG, Lee CH. ERK2-mediated phosphorylation of ZEB1 at S322 enhances PD-L1 expression and EMT, leading to pancreatic cancer progression. Cell communication and signaling : CCS, 23(1), 204 (2025) https://doi.org/10.1186/s12964-025-02182-3.
Yan C, Huang M, Li X, Wang T, Ling, R. (2019). Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocrine connections, 8(7), 988–996 (2019) https://doi.org/10.1530/EC-19-0246.
Obaid NM, Bedard K, Huang WY. Strategies for Overcoming Resistance in Tumours Harboring BRAF Mutations. International journal of molecular sciences, 18(3), 585 (2017) https://doi.org/10.3390/ijms18030585.
Chang KL, Lee MY, Chao WR, Han CP. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Human genomics, 10(1), 40 (2016) https://doi.org/10.1186/s40246-016-0096-9.
Liu X, Zhang P, Li C, Song X, Liu Z, Shao W, Li S, Wang X, Yu Z. Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer: a real-world retrospective study in China. Frontiers in oncology, 13, 1136380 (2023) https://doi.org/10.3389/fonc.2023.1136380.
Yang Y, Jin L, Li Y, Rao N, Gong C, Li S, Wu J, Zhao J, Ding L, Gan F, Zhang J, Feng R, Liu Z, Liu Q. Sequential neoadjuvant chemotherapy using pegylated liposomal doxorubicin and cyclophosphamide followed by taxanes with complete trastuzumab and pertuzumab treatment for HER2-positive breast cancer: A phase II single-arm study. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu, 36(1), 55–65 (2024) https://doi.org/10.21147/j.issn.1000-9604.2024.01.06
Li Y, Ganesan K, Chen J. Role of Biological Mediators of Tumor-Associated Macrophages in Breast Cancer Progression. Current medicinal chemistry, 29(33), 5420–5440 (2022) https://doi.org/10.2174/0929867329666220520121711
Li Z, Hu Y, Jones D, Zhao W, Tozbikian G, Parwani AV. Clinicopathologic Characteristics and Follow-Up Outcomes of Invasive Breast Carcinoma With Different Positive HER2 Fluorescence In Situ Hybridization Patterns: Experience From a Single Academic Institution. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 38(1), 100637 (2025) https://doi.org/10.1016/j.modpat.2024
Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-cancer agents in medicinal chemistry, 12(10), 1281–1305 (2012) https://doi.org/10.2174/187152012803833026.
Ying HZ, Yu CH, Chen HK, Zhang HH, Fang J, Wu F, Yu WY. Quinonoids: Therapeutic Potential for Lung Cancer Treatment. BioMed research international, 2460565 (2020) https://doi.org/10.1155/2020/2460565
Kang JH, Kang HS, Kim IK, Lee HY, Ha JH, Yeo CD, Kang HH, Moon HS, Lee SH. Curcumin sensitizes human lung cancer cells to apoptosis and metastasis synergistically combined with carboplatin. Experimental biology and medicine (Maywood, N.J.), 240(11), 1416–1425 (2015) https://doi.org/10.1177/1535370215571881.
Gavrilas LI, Cruceriu D, Mocan A, Loghin F, Miere D, Balacescu O. Plant-Derived Bioactive Compounds in Colorectal Cancer: Insights from Combined Regimens with Conventional Chemotherapy to Overcome Drug-Resistance. Biomedicines, 10(8), 1948 (2022) https://doi.org/10.3390/biomedicines10081948
Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng H, Leong MK, Sung PJ, Chen JC, Weng CF. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. British journal of pharmacology, 177(6),1409–1423 (2020) https://doi.org/10.1111/bph.14816
Dinu IM, Mihăilă M, Diculescu MM, Croitoru VM, Turcu-Stiolica A, Bogdan D, Miron MI, Lungulescu CV, Alexandrescu ST, Dumitrașcu T, Buică F, Luca IN, Lungulescu C, Negulescu MC, Gramaticu IM, Cazacu IM, Croitoru AE. Bevacizumab Treatment for Metastatic Colorectal Cancer in Real-World Clinical Practice. Medicina (Kaunas, Lithuania), 59(2), 350 (2023) https://doi.org/10.3390/medicina59020350.
Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, Boquet I, Tamberi S, Marmorino F, Moretto R, Ambrosini M, Tamburini E, Tortora G, Passardi, A, Bergamo F, Kassambara A, Sbarrato T, Morano F, Ritorto G, Borelli B. GONO Foundation Investigators. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. The Lancet. Oncology, 23(7), 876–887 (2022) https://doi.org/10.1016/S1470-2045(22)00274-1.
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nature reviews. Gastroenterology & hepatology, 16(6), 361–375 (2019) https://doi.org/10.1038/s41575-019-0126-x.
Duta-Ion SG, Juganaru IR, Hotinceanu IA, Dan A, Burtavel LM, Coman MC, Focsa IO Zaruha AG, Codreanu PC, Bohiltea LC, Radoi VE. Redefining Therapeutic Approaches in Colorectal Cancer: Targeting Molecular Pathways and Overcoming Resistance. International journal of molecular sciences, 25(23), 12507 (2024) https://doi.org/10.3390/ijms252312507.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal transduction and targeted therapy, 5(1), 22 (2020) https://doi.org/10.1038/s41392-020-0116-z.
Zhou D, Gulinuer W, Zhu N. Chemotherapy in combination with pembrolizumab and antiangiogenesis in young patients with advanced primary pulmonary mucinous adenocarcinoma: Two case reports. Science progress, 104(4), 368504211061971 (2021) https://doi.org/10.1177/00368504211061971.
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, Yang J. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. Journal of immunology research, 6984948 (2018) https://doi.org/10.1155/2018/6984948.
Rupp T, Genest L, Babin D, Legrand C, Hunault M, Froget G, Castagné, V. Anti-CTLA-4 and anti-PD-1 immunotherapies repress tumor progression in preclinical breast and colon model with independent regulatory T cells response. Translational oncology, 20, (2022) https://doi.org/10.1016/j.tranon.2022.101405.
Biegert, GWG, Rosewell SA, Suzuki M. Current development in adenoviral vectors for cancer immunotherapy. Molecular therapy oncolytics, 23, 571–581 (2021) https://doi.org/10.1016/j.omto.2021.11.014.
Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, Cogdill AP, Chen TJ, Song D, Scholler J, Kranz DM, Feldman MD, Young R, Keith B, Schreiber H, Clausen H, June CH. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity, 44(6), 1444–1454 (2016) https://doi.org/10.1016/j.immuni.2016.05.014.
Wagner S, Mullins CS, Linnebacher M. Colorectal cancer vaccines: Tumor-associated antigens vs neoantigens. World journal of gastroenterology, 24(48), 5418–5432 (2018) https://doi.org/10.3748/wjg.v24.i48.5418.
Yang, Chunhua DM. “Lipid-Based Drug Delivery Nanoplatforms for Colorectal Cancer Therapy.” Nanomaterials (Basel, Switzerland), 10,7 1424 (2020) https://doi.org/10.3390/nano10071424
Choukaife H, Seyam S, Alallam B, Doolaanea AA, Alfatama M. Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment. International journal of nanomedicine, 17, 3933–3966 (2022) https://doi.org/10.2147/IJN.S375229.
Nabi PN, Vahidfar N, Tohidkia MR, Hamidi AA, Omidi Y, Aghanejad A. Mucin-1 conjugated polyamidoamine-based nanoparticles for image-guided delivery of gefitinib to breast cancer. International journal of biological macromolecules, 174, 185–197 (2021) https://doi.org/10.1016/j.ijbiomac.2021.01.170.
Jain P, Kathuria H, Momin M. Clinical therapies and nano drug delivery systems for urinary bladder cancer. Pharmacology & therapeutics, 226, 107871 (2021) https://doi.org/10.1016/j.pharmthera.2021.107871.
Kumar A, Naik PK, Pradhan D, Ghosh G, Rath G. Mucoadhesive formulations: innovations, merits, drawbacks, and future outlook. Pharmaceutical development and technology, 25(7), 797–814 (2020) https://doi.org/10.1080/10837450.2020.1753771.
Yoon HY, Yang HM, Kim CH, Goo YT, Kang MJ, Lee S, Choi YW. Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer. Expert opinion on drug delivery, 17(11), 1555–1572 (2020) https://doi.org/10.1080/17425247.2020.1810016.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. The New England journal of medicine, 378(5), 449–459 (2018) https://doi.org/10.1056/NEJMoa1709919
Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, Kaltcheva T, Leong L, Morgan R, Chung V, Tinsley R, Eng M, Wilczynski S, Ellenhorn JDI, Diamond DJ, Cristea M. p53-Reactive T Cells Are Associated with Clinical Benefit in Patients with Platinum-Resistant Epithelial Ovarian Cancer After Treatment with a p53 Vaccine and Gemcitabine Chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(6), 1315–1325 (2018) https://doi.org/10.1158/1078-0432.CCR-17-2709.
Liao JB, Gwin WR, Urban RR, Hitchcock-Bernhardt KM, Coveler AL, Higgins DM, Childs JS, Shakalia HN, Swensen RE, Stanton SE, Tinker AV, Wahl TA, Ancheta RG, McGonigle KF, Dai JY, Disis ML, Goff BA. Pembrolizumab with low-dose carboplatin for recurrent platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer: survival and immune correlates. Journal for immunotherapy of cancer, 9(9), e003122 (2021) https://doi.org/10.1136/jitc-2021-003122.
Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, Li CW, Kim T, Chang SS, Lee HH, Hsu JL, Wang HL, Kuo CW, Chang WC, Hadad S, Purdie CA, McCoy AM, Cai S, Tu Y, Litton JK, Hung MC. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Molecular cell, 71(4), 606–620.e7 (2018) https://doi.org/10.1016/j.molcel.2018.07.030.
Stomper J, Rotondo JC, Greve G, Lübbert M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies. Leukemia, 35(7), 1873–1889 (2021) https://doi.org/10.1038/s41375-021-01218-0.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood, 133(1), 7–17 (2019) https://doi.org/10.1182/blood-2018-08-868752.
Becker H, Pfeifer D, Ihorst G, Pantic M, Wehrle J, Rüter BH, Bullinger L, Hackanson B, Germing U, Kuendgen A, Platzbecker U, Döhner K, Ganser A, Hagemeijer A, Wijermans PW, Döhner H, Duyster J, Lübbert M. Monosomal karyotype and chromosome 17p loss or TP53 mutations in decitabine-treated patients with acute myeloid leukemia. Annals of hematology, 99(7), 1551–1560 (2020) https://doi.org/10.1007/s00277-020-04082-7.
Koutsounas I, Giaginis C, Patsouris E, Theocharis S. Current evidence for histone deacetylase inhibitors in pancreatic cancer. World journal of gastroenterology, 19(6), 813–828 (2013) https://doi.org/10.3748/wjg.v19.i6.813
Sim W, Lim WM, Hii LW, Leong CO, Mai CW. Targeting pancreatic cancer immune evasion by inhibiting histone deacetylases. World journal of gastroenterology, 28(18), 1934–1945 (2022) https://doi.org/10.3748/wjg.v28.i18.1934.
Maietta I, Martínez-Pérez A, Álvarez R, De Lera ÁR, González-Fernández Á, Simón-Vázquez R. Synergistic Antitumoral Effect of Epigenetic Inhibitors and Gemcitabine in Pancreatic Cancer Cells. Pharmaceuticals (Basel, Switzerland), 15(7), 824 (2022) https://doi.org/10.3390/ph15070824.
Bouyahya A, El Omari N, Bakha M, Aanniz T, El Menyiy N, El Hachlafi N, El Baaboua A, El-Shazly M, Alshahrani MM, Al Awadh AA, Lee LH, Benali T, Mubarak MS. Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review. Pharmaceuticals (Basel, Switzerland), 15(10), 1235 (2022) https://doi.org/10.3390/ph15101235
Gordon MS, Shapiro GI, Sarantopoulos J, Juric D, Lu B, Zarotiadou A, Connarn JN, Le Bruchec Y, Dumitru CD, Harvey RD. Phase Ib Study of the Histone Deacetylase 6 Inhibitor Citarinostat in Combination With Paclitaxel in Patients With Advanced Solid Tumors. Frontiers in oncology, 11, 786120 (2022) https://doi.org/10.3389/fonc.2021.786120.
Adamska A, Domenichini A, Capone E, Damiani V, Akkaya BG, Linton KJ, Di SP, Chen X, Keeton AB, Ramirez-Alcantara V, Maxuitenko Y, Piazza GA, De LV, Sala G, Falasca M. Pharmacological inhibition of ABCC3 slows tumour progression in animal models of pancreatic cancer. Journal of experimental & clinical cancer research : CR, 38(1), 312 (2019) https://doi.org/10.1186/s13046-019-1308-7
Koutsaki M, Spandidos DA, Zaravinos A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: prognostic value and prospective role in ovarian cancer therapeutics. Cancer letters, 351(2), 173–181 (2014) https://doi.org/10.1016/j.canlet.2014.05.022.
Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines, 9(4), 359 (2021) https://doi.org/10.3390/vaccines9040359
Patel MN, Lakkadwala S, Majrad MS, Injeti ER, Gollmer SM, Shah ZA, Boddu SH, Nesamony J. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS PharmSciTech, 15(6), 1498–1508 (2014) https://doi.org/10.1208/s12249-014-0168-x.
Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, Xu C, Merlin D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Molecular therapy : the journal of the American Society of Gene Therapy, 24(10), 1783–1796 (2016) https://doi.org/10.1038/mt.2016.159.
Udofot O, Affram K, Israel B, Agyare, E. Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines. Integrative cancer science and therapeutics, 2(5), 245–252 (2015) https://doi.org/10.15761/icst.1000150
Bhaskaran NA, Jitta SR, Salwa CS, Kumar N, Kumar L. Orally delivered solid lipid nanoparticles of irinotecan coupled with chitosan surface modification to treat colon cancer: Preparation, in-vitro and in-vivo evaluations. International journal of biological macromolecules, 211, 301–315 (2022) https://doi.org/10.1016/j.ijbiomac.2022.05.060.
Levink I, Bruno MJ, Cahen DL. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Current treatment options in gastroenterology, 16(3), 316–332 (2018) https://doi.org/10.1007/s11938-018-0190-2
Published
How to Cite
Issue
Section
Copyright (c) 2025 Kaniga Pandi, Binoy Varghese Cheriyan, Vishali Ramesh, Sowparnika Murugavel, Jaya Surya Venkatesan

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















