Niosomes: an extensive analysis of its structure, preparation, and uses in drug delivery
DOI:
https://doi.org/10.69857/joapr.v13i3.1000Keywords:
Niosomes, Vesicular, Hydrophilic, Lipids, VehiclesAbstract
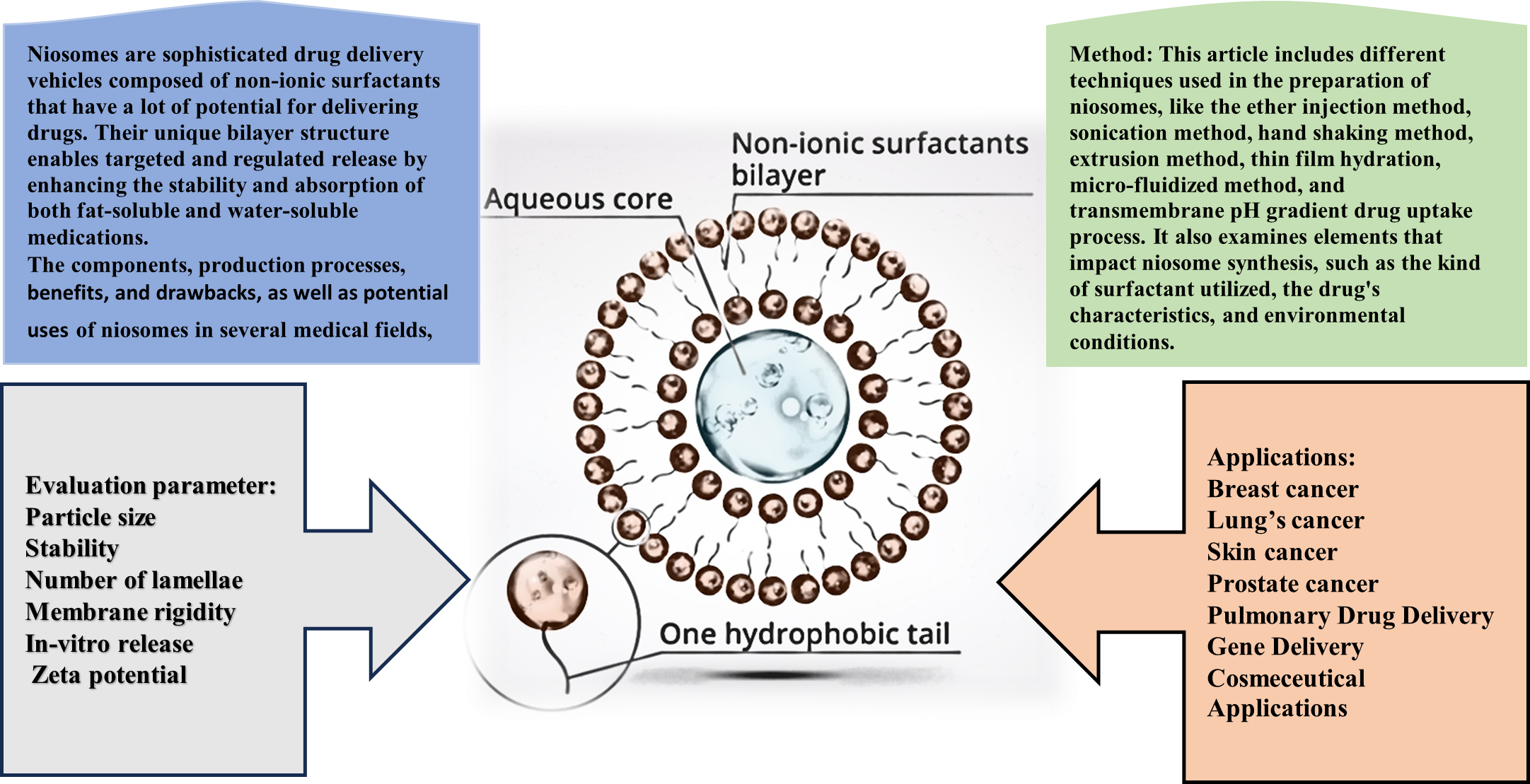
Background: Niosomes are sophisticated drug delivery vehicles composed of non-ionic surfactants that have a lot of potential for delivering drugs. Their unique bilayer structure enables the targeted and regulated release of both fat-soluble and water-soluble medications, enhancing their stability and absorption. This review discusses the components, production processes, benefits, drawbacks, and potential uses of niosomes in various medical fields, including cutaneous medication delivery and cancer treatment. Objective: This article provides an in-depth overview of niosomes as novel drug delivery vehicles, highlighting their benefits, preparation techniques, applications, and assessment criteria. It aims to highlight how they can improve therapeutic efficacy and bioavailability across various medicinal domains. Methodology: This article outlines multiple techniques employed in the preparation of niosomes, including the ether injection method, sonication method, hand-shaking method, extrusion method, thin film hydration, microfluidization method, and Transmembrane pH gradient drug uptake process. It also examines elements that impact niosome synthesis, such as the kind of surfactant utilized, the drug's characteristics, and environmental conditions. Conclusion: Ongoing research is focused on enhancing niosomal formulations and expanding their use in clinical settings, despite challenges such as instability and a short shelf life. Overall, niosomes are a significant development in drug delivery technology, with the potential to enhance treatment effectiveness and improve patient care.
Downloads
References
Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER. Pharmaceutical nanotechnology: From the bench to the market. Future J Pharm Sci., 8, 12 (2022) https://doi.org/10.1186/s43094-022-00400-0
Sahani S, Sharma YC. Advancements in applications of nanotechnology in global food industry. Food Chem, 342,128318 (2021) https://doi.org/10.1016/j.foodchem.2020.128318
Thatyana M, Dube NP, Kemboi D, Manicum ALE, Mokgalaka-Fleischmann NS, Tembu JV. Advances in phytonanotechnology: A plant-mediated green synthesis of metal nanoparticles using Phyllanthus plant extracts and their antimicrobial and anticancer applications. Nanomaterials, 13, 2616 (2023) https://doi.org/10.3390/nano13192616
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules, 25, 112 (2020) https://doi.org/10.3390/molecules25010112
Ansari MF, Aftab Alam. Niosomes as novel drug delivery system: Review article. Int J Pharm Res Appl, 7(1), 171-8 (2022) https://doi.org/10.35629/7781-0701171178
Tariq F, Zaman M, Waqar MA, Saeed MA, Sarfraz RM. Design, Optimization & Characterization of Niosomal & Polymeric Nanoparticles. Int. J. Polym. Mater. Polym. Biomater, 73, 1353–1366 (2024) https://doi.org/10.1080/00914037.2023.2277235
Momekova DB, Gugleva, VE, Petrov PD. Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery. ACS Omega, 6, 33265–33273 (2021) https://doi.org/10.1021/acsomega.1c05083
Junyaprasert VB, Teeranachaideekul V, Supaperm T. Effect of Charged and Non-ionic Membrane Additives on Physicochemical Properties and Stability of Niosomes. AAPS PharmSciTech, 9, 851-9 (2008) https://doi.org/10.1208/s12249-008-9121-1
Moammeri A, Chegeni, MM, Sahrayi H, Ghafelehbashi R, Memarzadeh F, Mansouri A, Akbarzadeh I, Abtahi MS, Hejabi F, Ren Q. et al. Current Advances in Niosomes Applications for Drug Delivery and Cancer Treatment. Mater. Today. Bio, 23, 100837 (2023) https://doi.org/10.1016/j.mtbio.2023.100837
Riccardi D, Baldino L, Reverchon E. Liposomes, Transfersomes and Niosomes: production Methods and Their Applications in the Vaccinal Field. J. Transl. Med, 22, 339 (2024) https://doi.org/10.1186/s12967-024-05160-4
Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: A review on niosomal research in the last decade. J Drug Deliv Sci Technol, 56, 101581(2020) https://doi.org/10.1016/j.jddst.2020.101581
Aparajay P, Dev A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur J Pharm Sci, 168,106052 (2022) https://doi.org/10.1016/j.ejps.2021.106052
Izhar MP, Hafeez A, Kushwaha P, Simrah. Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications. J. Clust. Sci, 34, 2257–2273 (2023) https://doi.org/10.1007/s10876-023-02423-w
Mawazi SM, Ann TJ, Widodo RT. Application of Niosomes in Cosmetics: A Systematic Review. Cosmetics, 9, 127 (2022) https://doi.org/10.3390/cosmetics9060127
Barani M, Hajinezhad MR, Zargari F, Shahraki S, Davodabadi F, Mirinejad S, Sargazi S, Rahdar A, Díez-Pascual AM. Preparation, Characterization, Cytotoxicity and Pharmacokinetics of Niosomes Containing Gemcitabine: In Vitro, in Vivo, and Simulation Studies. J. Drug Delivery Sci. Technol, 84, 104505 (2023) https://doi.org/10.1016/j.jddst.2023.104505
Zaer M, Moeinzadeh A, Abolhassani H, Rostami N, Tavakkoli Yaraki M, Seyedim SA, Nabipoorashrafi SA, Bashiri Z, Moeinabadi-Bidgoli K, Moradbeygi F. et al. Doxorubicin-Loaded Niosomes Functionalized with Gelatine and Alginate as pH-Responsive Drug Delivery System: A 3D Printing Approach. Int. J. Biol. Macromol, 253, 126808 (2023) https://doi.org/10.1016/j.ijbiomac.2023.126808
Ghasemiyeh P, Moradishooli F, Daneshamouz S, Heidari R, Niroumand U, Mohammadi-Samani S. Optimization, Characterization, and Follicular Targeting Assessment of Tretinoin and Bicalutamide Loaded Niosomes. Sci. Rep, 13, 20023 (2023) https://doi.org/10.1038/s41598-023-47302-6
Arslanov VV, Ermakova EV, Krylov DI, Popova OO. On the Relationship between the Properties of Planar Structures of Non-Ionic Surfactants and Their Vesicular Analogues–Niosomes. J. Colloid Interf. Sci, 640, 281–295 (2023) https://doi.org/10.1016/j.jcis.2023.02.110
Nerli G, Robla S, Bartalesi M, Luceri C, D’Ambrosio M, Csaba N, Maestrelli F. Chitosan Coated Niosomes for Nose-to-Brain Delivery of Clonazepam: Formulation, Stability and Permeability Studies. Carbohydrate Polym. Tech. Appl, 6, 100332 (2023) https://doi.org/10.1016/j.carpta.2023.100332
Masjedi M, Montahaei T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: fabrication, characterization, pharmaceutical, and cosmetic applications. J Drug Deliv Sci Technol, 61,102234 (2021) https://doi.org/10.1016/j.jddst.2020.102234
Sharma S, Garg A, Agrawal R, Chopra H, Pathak D. A Comprehensive Review on Niosomes as a Tool for Advanced Drug Delivery. Pharm. Nanotechnol, 12, 206–228 (2024) https://doi.org/10.2174/2211738511666230726154557
Fadaei MS, Fadaei MR, Kheirieh AE, Rahmanian-Devin P, Dabbaghi MM, Nazari Tavallaei K, Shafaghi A, Hatami H, Baradaran Rahimi V, Nokhodchi A, et al. Niosome as a promising tool for increasing the effectiveness of anti-inflammatory compounds. Excli J, 23, 212–263 (2024) https://doi.org/10.17179/excli2023-6868
Lin YK, Hsiao CY, Alshetaili A, Aljuffali IA, Chen EL, Fang JY. Lipid-based nanoformulation optimization for achieving cutaneous targeting: niosomes as the potential candidates to fulfill this aim. Eur J Pharm Sci, 186, 106458 (2023) https://doi.org/10.1016/j.ejps.2023.106458
Sayyad N, Maji R, Omolo CA, Ibrahim UH, Pathan TK, Devnarain N, et al. Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension. International Journal of Pharmaceutics, 609, 121191 (2021) https://doi.org/10.1016/j.ijpharm.2021.121191
Durga B, Veera L. Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): a brief review of these in enhancing transdermal delivery of drug. Futur J Pharm Sci, 6, 100 (2020) https://doi.org/10.1186/s43094-020-00117-y
Sun Z. Optimization of clobetasol propionate loaded niosomal gel for the treatment of psoriasis: and efficacy study. J. Dispers. Sci. Technol, 44(14), 2599-609 (2023) https://doi.org/10.1080/01932691.2022.2110111
Thabet Y, Elsabahy M, Eissa NG. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods, 9, 199 (2022) https://doi.org/10.1016/j.ymeth.2021.05.004
Arslanov VV, Krylov DI. Reassembly of the Vesicular Structure of Niosomes after Their Destruction in a Mechanical Field. J. Colloid Interf. Sci, 662, 342–356 (2024) https://doi.org/10.1016/j.jcis.2024.02.035
Umbarkar M, Thakare S, Surushe T, Giri A, Chopade V. Formulation and evaluation of liposome by thin film hydration method. J. Drug Deliv. Ther, 11(1), 72-6 (2021) https://doi.org/10.22270/jddt.v11i1.4677
Sharma S, Kumari N, Garg D, ChauhanmSA. Compendium of Bioavailability Enhancement via Niosome Technology. Pharm. Nanotechnol, 11, 324–338 (2023) https://doi.org/10.2174/2211738511666230309104323
Albash R, Al-Mahallawi AM, Hassan M, Alaa-Eldin AA. Optimization of niosomal gel for enhanced topical delivery of fusidic acid: in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol., 67, 102950, (2022) https://doi.org/10.1016/j.jddst.2021.102950
Katkale, Akshay, Review on niosomes as novel drug delivery system. World J. Pharm. Res,1137 (2022) https://doi.org/10.20959/wjpr20223 23338
Moammeri A, Chegeni MM, Sahrayi H, Ghafelehbashi R, Memarzadeh F, Mansouri A, et al. Current advances in niosomes applications for drug delivery and cancer treatment. Materials Today Bio,100837 (2023) https://doi.org/10.1016/j.mtbio.2023.100837
Yasamineh S, Yasamineh P, Kalajahi HG, Gholizadeh O, Yekanipour Z, Afkhami H, et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm, 624,121878 (2022) https://doi.org/10.1016/j.ijpharm.2022.121878
Arslanov VV, Ermakova EV, Krylov DI, Popova OO. On the relationship between the properties of planar structures of non-ionic surfactants and their vesicular analogues–niosomes. J Colloid Interface Sci, 640, 281-295 (2023) https://doi.org/10.1016/j.jcis.2023.02.110
John CR, Sailaja AK. A curcumin loaded niosomes as novel drug delivery system by ether injection method. Drug Discovery, 17, e19dd1918 (2023) https://doi.org/10.54905/disssi.v17i39.e19dd1918
Ge X, Wei M, He S, Yuan WE. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics, 11(2), 55 (2019) https://doi.org/10.3390/pharmaceutics11020055
Chen S, Hanning S, Falconer J, Locke M, Wen J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. European journal of pharmaceutics and biopharmaceutics, 144, 18-39 (2019) https://doi.org/10.1016/j.ejpb.2019.08.015
Reddy KT, Gandla K. Novel Vesicular Drug Delivery Systems Proniosomes. Pharm Res, 6(3), 000272 (2022) https://doi.org/10.23880/oajpr-16000272
Rajeev J, Kamalasanan K, Sapa H, Sabitha M, Abhi C. Controlled release nanomedicine (CRNM) of aspirin using “biomimetic niosomal nanoparticles (BNNs)” for Covid-19 and cardiovascular treatment: DOE based optimization. OpenNano, 9, 100119 (2023) https://doi.org/10.1016/j.onano.2023.100119
Sankhyan A, Pawar P. Recent trends in niosome as vesicular drug delivery system. J Appl Pharm Sci, 2(6), 28-30 (2012) https://doi.org/10.7324/JAPS.2012.2625
Kietrungruang K, Sookkree S, Sangboonruang S, Semakul N, Poomanee W, Kitidee K, Tragoolpua Y, Tragoolpua K. Ethanolic extract propolis-loaded niosomes diminish phospholipase B1, biofilm formation, and intracellular replication of Cryptococcus neoformans in macrophages. Molecules, (2023) https://doi.org/10.3390/molecules28084215
Sharma R, Dua J, Parsad D. An overview on niosomes: novel pharmaceutical drug delivery system. J Drug Delivery Ther, (2022) https://doi.org/10.22270/jddt.v12i2 s.5264
Elmowafy E, El-Derany MO, Biondo F, Tiboni M, Casettari L, Soliman ME, et al. Quercetin loaded monolaurate sugar esters-based niosomes: Sustained release and mutual antioxidant—hepatoprotective interplay. Pharmaceutics, 12(2), 143 (2020) https://doi.org/10.3390/pharmaceutics12020143
Ramkanth S, Chetty CM, Sudhakar Y, Thiruvengadarajan VS, Anitha P, et al. Development, characterization & in vivo evaluation of proniosomal based transdermal delivery system of Atenolol. Futur J Pharm Sci, 4, 80-7 (2018) https://doi.org/10.1016/j.fjps.2017.10.003
Firozian F, Karami S, Ranjbar A, Azandaryani MT, Nili-Ahmadabadi A. Improvement of therapeutic potential N-acetylcysteine in acetaminophen hepatotoxicity by encapsulation in PEGylated nano-niosomes. Life Sciences, 255, 117832 (2020) https://doi.org/10.1016/j.fjps.2017.10.003
Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci, 8, 12 (2022) https://doi.org/10.1186/s43094-022-00400-0
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules, 25, 112 (2020) https://doi.org/10.3390/molecules25010112
Soni RA, Rizwan MA, Singh, S. Opportunities and potential of green chemistry in nanotechnology. Nanotechnol. Environ. Eng, 7, 661–673 (2022) https://doi.org/10.1007/s41204-022-00233-5
Teron C, Bhuyan A, Saikia P, Dutta SR, Gogoi H, Rongpi S. A comprehensive review on niosomes in drug delivery and recent advancements. J Drug Deliv Ther. 14 (6) (2024) 262–273. https://doi.org/10.22270/jddt.v14i6.6651
Abruzzo A, Pucci R, Abruzzo PM, Canaider S, Parolin C, Vitali B, Valle F, Brucale M, Cerchiara T, Luppi B, et al. Azithromycin-loaded liposomes and niosomes for the treatment of skin infections: influence of excipients and preparative methods on the functional properties. Eur J Pharm Biopharm, 197, 114233 (2024) https://doi.org/10.1016/j.ejpb.2024.114233
Poustforoosh A. Investigation on the structural and dynamical properties of cationic, anionic, and catanionic niosomes as multifunctional controlled drug delivery system for cabozantinib. Colloids Surf A, 687,133547 (2024) https://doi.org/10.1016/j.molliq.2024.125338
Owodeha-Ashaka K, Ilomuanya MO, Iyire A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Prog Biomater, 10, 207–20 (2021) https://doi.org/10.1007/s40204-021-00164-5
Published
How to Cite
Issue
Section
Copyright (c) 2025 Vaishali Sharma, Chinkey Mittal, Shweta Shekhedwal, Vasu Chaudhary, Sachin Kumar

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















