Cardioprotective effects of Anthocephalus cadamba bark extract and pyridoxine in STZ-induced diabetic rats
DOI:
https://doi.org/10.69857/joapr.v14i1.1735Keywords:
Anthocephalus cadamba, STZ-induced diabetic rats, Diabetes mellitus, Oxidative stress markers, cardiomyopathy, triglyceridesAbstract
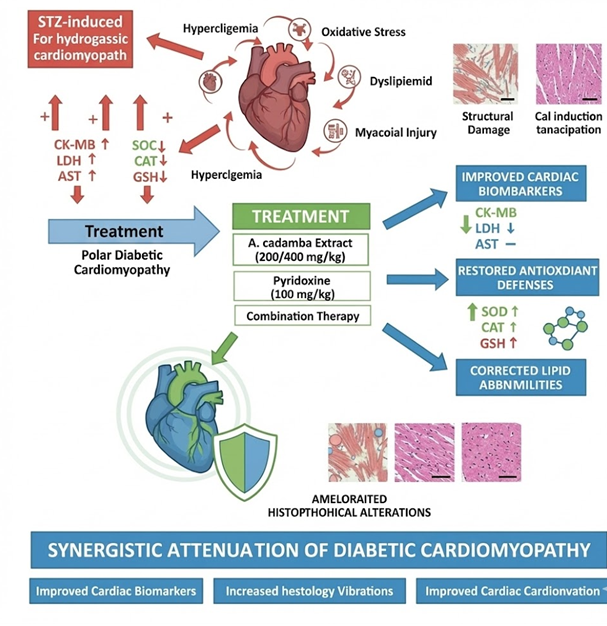
Background: Oxidative stress driven by hyperglycaemia and hyperlipidaemia plays a major role in diabetic cardiomyopathy. Although Anthocephalus cadamba bark and pyridoxine have traditional uses, their combined efficacy against diabetic cardiomyopathy remains underexplored. In this work, streptozotocin (STZ)-induced type 2 diabetic rats were used to investigate the preventive benefits of an ethanolic bark extract of A. cadamba given either with or without pyridoxine. Methods: Diabetes was induced in Wistar rats with STZ (45 mg/kg, i.p.). Animals were allocated to eight groups and treated with metformin, A. cadamba extract (200 or 400 mg/kg), pyridoxine (100 mg/kg), or combinations of these agents for eight weeks. Biochemical markers (CK-MB, LDH, AST), lipid profile, and oxidative stress enzymes were assessed, along with heart histopathology. Results: Diabetic rats showed marked elevations in CK-MB, LDH, and AST, which were significantly reduced by A. cadamba (200–400 mg/kg) (e.g., CK-MB, LDH, and AST decreased by approximately 25-33%). (p < 0.001). Combining pyridoxine with other therapies produced the strongest effect, lowering CK-MB, LDH, and AST by 30–41% (p < 0.001). Treatment with A. cadamba extract alone and in combination with pyridoxine significantly improved dyslipidaemia, with HDL rising from ~42 to 52% (p < 0.01-0.001) and decreasing serum-lipoproteins concentration (total Cholesterol, triglyceride, LDL, and VLDL) by ~40 to 55% (p < 0.01). Antioxidant defenses were also restored, with SOD, CAT, and GSH levels rising by 70–80% (p < 0.05–0.001). Histology confirmed reduced necrosis and fibre degeneration, most notably in the combination-treated groups. Conclusion: A. cadamba extract and pyridoxine, particularly in combination, mitigate oxidative stress, hyperlipidaemia, and cardiac injury in STZ-induced diabetic rats, suggesting therapeutic potential against diabetic cardiomyopathy.
Downloads
References
Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Heal. Sci. Reports, 7, e2004 (2024) https://doi.org/10.1002/HSR2.2004.
Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes, 6, 1246 (2015) https://doi.org/10.4239/WJD.V6.I13.1246.
Abdullah AR, Seliem MA, Khidr EG, Sobhy AM, El-Shiekh RA, Hafeez MSA El, El-Husseiny AA. A comprehensive review on diabetic cardiomyopathy (DCM): histological spectrum, diagnosis, pathogenesis, and management with conventional treatments and natural compounds. Naunyn. Schmiedebergs. Arch. Pharmacol., 398, 9929 (2025) https://doi.org/10.1007/S00210-025-03980-9.
Ghasemi A, Jeddi S. Streptozotocin as a tool for induction of rat models of diabetes: a practical guide. EXCLI J., 22, 274 (2023) https://doi.org/10.17179/EXCLI2022-5720.
D’Oria R, Schipani R, Leonardini A, Natalicchio A, Perrini S, Cignarelli A, Laviola L, Giorgino F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev., 2020, 5732956 (2020) https://doi.org/10.1155/2020/5732956.
Liu M, Lv J, Pan Z, Wang D, Zhao L, Guo X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front. Cardiovasc. Med., 9, 945142 (2022) https://doi.org/10.3389/FCVM.2022.945142.
Riaz M, Khalid R, Afzal M, Anjum F, Fatima H, Zia S, Rasool G, Egbuna C, Mtewa AG, Uche CZ, Aslam MA. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr., 11, 2500 (2023) https://doi.org/10.1002/FSN3.3308.
Kaushik S, Ali Z, Tangri P, Bhatt B, Devi A.Pharmacological activities of anthocephalus cadamba: a concise review. Int. J. Biol. Pharm. Allied Sci., 10, 916-24 (2021) https://doi.org/10.31032/IJBPAS/2021/10.3.5402.
Sukumaran V, Gurusamy N, Yalcin HC, Venkatesh S. Understanding diabetes-induced cardiomyopathy from the perspective of renin angiotensin aldosterone system. Pflugers Arch., 474, 63 (2021) https://doi.org/10.1007/S00424-021-02651-X.
Parra M, Stahl S, Hellmann H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells, 7, 84 (2018) https://doi.org/10.3390/CELLS7070084.
D’Haese S, Claes L, Jaeken E, Deluyker D, Evens L, Heeren E, Haesen S, Vastmans L, Lambrichts I, Wouters K, Schalkwijk CG, Hansen D, Eijnde BO, Bito V. Pyridoxamine Alleviates Cardiac Fibrosis and Oxidative Stress in Western Diet-Induced Prediabetic Rats. Int. J. Mol. Sci., 25, 8508 (2024) https://doi.org/10.3390/IJMS25158508/S1.
Mutavdzin Krneta S, Gopcevic K, Stankovic S, Jakovljevic Uzelac J, Todorovic D, Labudovic Borovic M, Rakocevic J, Djuric D. Insights into the Cardioprotective Effects of Pyridoxine Treatment in Diabetic Rats: A Study on Cardiac Oxidative Stress, Cardiometabolic Status, and Cardiovascular Biomarkers. Diagnostics, 14, 1507 (2024) https://doi.org/10.3390/DIAGNOSTICS14141507.
Qnais E, Gammoh O, Bsieso Y, Alqudah M, Wedyan M, Altaber S, Aljabali AAA, Alqudah A, Hatahet T. Scopoletin as a cardioprotective agent against cisplatin-induced oxidative stress and inflammation. Phytomedicine Plus, 5, 100738 (2025) https://doi.org/10.1016/J.PHYPLU.2025.100738.
Shrivastav D, Kumbhakar SK, Srivastava S, Singh DD. Natural product-based treatment potential for type 2 diabetes mellitus and cardiovascular disease. World J. Diabetes, 15, 1603 (2024) https://doi.org/10.4239/WJD.V15.I7.1603.
Chandrashekar KS, Abinash B, Prasanna KS. Anti-inflammatory effect of the methanol extract from Anthocephalus cadamba stem bark in animal models. Int. J. Plant Biol., 1, 30–2 (2010) https://doi.org/10.4081/pb.2010.e6.
Konyanee A, Chaniad P, Phuwajaroanpong A, Plirat W, Viriyavejakul P, Septama AW, Punsawad C. Exploring the potential antimalarial properties, safety profile, and phytochemical composition of Mesua ferrea Linn. PLoS One, 19, e0312047 (2024) https://doi.org/10.1371/JOURNAL.PONE.0312047.
Ettlin RA, Kuroda J, Plassmann S, Prentice DE. Successful Drug Development Despite Adverse Preclinical Findings Part 1: Processes to Address Issues and Most Important Findings. J. Toxicol. Pathol., 23, 189 (2010) https://doi.org/10.1293/TOX.23.189.
Emmer KM, Russart GKL, Walker WH, Nelson RJ, Courtney DeVries A. Effects of Light at Night on Laboratory Animals and Research Outcomes. Behav. Neurosci., 132, 302 (2018) https://doi.org/10.1037/BNE0000252.
Thangaraj P. Evaluation of Anti-diabetic Property on Streptozotocin-Induced Diabetic Rats. Prog. Drug Res., 71, 145–9 (2016) https://doi.org/10.1007/978-3-319-26811-8_24.
Qamar F, Sultana S, Sharma M. Animal models for induction of diabetes and its complications. J. Diabetes Metab. Disord., 22, 1021 (2023) https://doi.org/10.1007/S40200-023-01277-3.
Singh SK, Kesari AN, Gupta RK, Jaiswal D, Watal G. Assessment of antidiabetic potential of Cynodon dactylon extract in streptozotocin diabetic rats. J. Ethnopharmacol., 114, 174–9 (2007) https://doi.org/10.1016/J.JEP.2007.07.039.
Van Herck H, Baumans V, Brandt CJWM, Boere HAG, Hesp APM, Van Lith HA, Schurink M, Beynen AC. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab. Anim., 35, 131–9 (2001) https://doi.org/10.1258/0023677011911499.
Djakpo DK, Wang ZQ, Shrestha M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci. – Atheroscler. Dis., 5, 279–83 (2020) https://doi.org/10.5114/AMSAD.2020.103028.
Chan FKM, Moriwaki K, De Rosa MJ. Detection of Necrosis by Release of Lactate Dehydrogenase (LDH) Activity. Methods Mol. Biol., 979, 65 (2013) https://doi.org/10.1007/978-1-62703-290-2_7.
Al-Hadi HA, Fox KA. Cardiac Markers in the Early Diagnosis and Management of Patients with Acute Coronary Syndrome. Sultan Qaboos Univ. Med. J., 9, 231 (2009) https://doi.org/10.18295/2075-0528.2796.
El-Nasr NMEA, Hussien YA, El-Baset MA, Shabana ME, Saleh DO. Astaxanthin mitigates diabetic cardiomyopathy and nephropathy in HF/HFr/STZ diabetic rats via modulating NOX4, fractalkine, Nrf2, and AP-1 pathways. Sci. Rep., 15, 20199 (2025) https://doi.org/10.1038/S41598-025-06263-8.
Wang X, Zhao D, Farnell MB, Milby AC, Archer GS, Peebles ED, Gurung S. Evaluation of Euthanasia Methods on Behavioral and Physiological Responses of Newly Hatched Male Layer Chicks. Anim. 2021, Vol. 11, Page 1802, 11, 1802 (2021) https://doi.org/10.3390/ANI11061802.
Varesi A, Campagnoli LIM, Carrara A, Pola I, Floris E, Ricevuti G. Non-Enzymatic Antioxidants against Alzheimer's Disease: Prevention, Diagnosis and Therapy. Antioxidants (Basel), 12(1), 180 (2023) https://doi.org/10.3390/antiox12010180
Yilgor A, Demir C. Determination of oxidative stress level and some antioxidant activities in refractory epilepsy patients. Sci. Rep., 14, 6688 (2024) https://doi.org/10.1038/S41598-024-57224-6.
Zacharis CK, Tzanavaras PD. Liquid chromatography coupled to on-line post column derivatization for the determination of organic compounds: A review on instrumentation and chemistries. Anal. Chim. Acta, 798, 1–24 (2013) https://doi.org/10.1016/J.ACA.2013.07.032.
Van Noorden CJF, Butcher RG. The involvement of superoxide anions in the nitro blue tetrazolium chloride reduction mediated by NADH and phenazine methosulfate. Anal. Biochem., 176, 170–4 (1989) https://doi.org/10.1016/0003-2697(89)90288-1.
Hadwan MH. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem., 19, (2018) https://doi.org/10.1186/S12858-018-0097-5.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem., 195, 133–40 (1952) https://doi.org/10.1016/s0021-9258(19)50881-x.
Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol., 97, 2499 (2023) https://doi.org/10.1007/S00204-023-03562-9.
Komolafe OA, Adeyemi DO, Adewole SO, Obuotor EM. Streptozotocin-induced diabetes alters the serum lipid profiles of adult wistar rats. Internet J. Cardiovasc. Res., 7, (2010) https://doi.org/10.5580/2251.
Kannan S, Mahadevan S, Ramji B, Jayapaul M, Kumaravel V. LDL-cholesterol: Friedewald calculated versus direct measurement-study from a large Indian laboratory database. Indian J. Endocrinol. Metab., 18, 502 (2014) https://doi.org/10.4103/2230-8210.137496.
Sajja A, Park J, Sathiyakumar V, Varghese B, Pallazola VA, Marvel FA, et al. Comparison of Methods to Estimate Low-Density Lipoprotein Cholesterol in Patients With High Triglyceride Levels. JAMA Netw. Open, 4, e2128817 (2021) https://doi.org/10.1001/JAMANETWORKOPEN.2021.28817.
Rathi H, Kumar R, Goyal B, Kant R, Mirza AA, Rana S, Naithani M. Assessment of Dyslipidemia, Lipid Ratios, and Atherogenic Indices as Cardiovascular Risk Factors in Prediabetic and Diabetic Subjects. J. Lab. Physicians, 14, 420 (2022) https://doi.org/10.1055/S-0042-1744240.
Baskin DG. Fixation and Tissue Processing in Immunohistochemistry. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech., 3797–806 (2014) https://doi.org/10.1016/B978-0-12-386456-7.07402-5.
Shah A, Kulkarni D, Ingale Y, Koshy A, Bhagalia S, Bomble N. Kerosene: Contributing agent to xylene as a clearing agent in tissue processing. J. Oral Maxillofac. Pathol., 21, 367 (2017) https://doi.org/10.4103/JOMFP.JOMFP_14_15.
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc., 2008, (2008) https://doi.org/10.1101/PDB.PROT4986.
Ferreira D, Vale J, Curado M, Polónia A, Eloy C. The impact of different coverslipping methods in the quality of the whole slide images used for diagnosis in pathology. J. Pathol. Inform., 13, 100098 (2022) https://doi.org/10.1016/J.JPI.2022.100098.
Lovitt RW, Wright CJ. Microscopy: Light Microscopy. Encycl. Food Microbiol. Second Ed., 684–92 (2014) https://doi.org/10.1016/B978-0-12-384730-0.00213-5.
Xuan L, Ju Z, Skonieczna M, Zhou PK, Huang R. Nanoparticles‐induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm, 4, e327 (2023) https://doi.org/10.1002/MCO2.327.
Published
How to Cite
Issue
Section
Copyright (c) 2026 Talever Singh, Saravanan K

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















