Review on advancements in liposomal dosage forms with newer methodologies and clinical perspectives
DOI:
https://doi.org/10.69857/joapr.v14i1.1302Keywords:
Liposomes, lipid bilayer, encapsulation, method of preparation, therapeutic efficacyAbstract
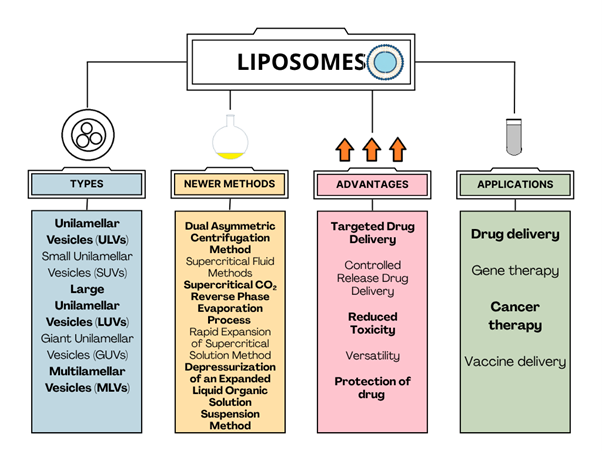
Background: Liposomes are widely used as drug delivery systems because of their reduced systemic toxicity. Over the past few decades, numerous drug-loaded liposomes have been approved for clinical use in the treatment of cancer, viral, and fungal infections. Various liposomal formulations have progressed to later phases of clinical trials. Liposomes are spherical vesicles composed of a single or multiple phospholipid bilayers surrounding an aqueous core. Drug-loaded liposomes can exhibit controlled or targeted drug delivery, low immunogenicity, high biocompatibility, biodegradability, prolonged drug half-life, increased efficiency, reduced systemic toxicity, and enhanced pharmacokinetic properties. Methodology: This review article addresses the characteristics and types of liposomes; novel methods for their preparation, such as the Supercritical Anti-solvent Method and the Dual Asymmetric Centrifugation Method; lipid preferences; future directions for liposomes; marketed liposomal formulations; and associated patents. Results and Discussion: It has the potential to protect the drug against degradation. The aforementioned drug delivery system increases in vivo drug distribution toward target sites. PEGylated liposomes can prolong circulation time. It requires expertise in techniques, such as thin-film hydration and reverse-phase evaporation, for preparation. It has been utilized in nanomedicine. This particular delivery system requires characterizations like size, drug loading, drug release, etc. Conclusion: Liposome-embedded delivery systems advance nanotechnology and biopharmaceutics. The role of modern medicine has continued to expand, particularly in the management of chronic diseases.
Downloads
References
Medina-Alarcon KP, Voltan AR, Fonseca-Santos B, Moro IJ, de Oliveira Souza F, Chorilli M, Fusco-Almeida AM. Highlights in nanocarriers for the treatment against cervical cancer. Mater Sci Eng C, 80, 748–759 (2017) https://doi.org/10.1016/j.msec.2017.07.021.
Panwar P, Kumar S, Chand P, Chauhan AS, Jakhmola V. Nanostructured lipid carriers (NLCs): A comprehensive review of drug delivery advancements. Journal of Applied Pharmaceutical Research, 13, 20–38 (2025) https://doi.org/10.69857/joapr.v13i2.676.
Rommasi F, Esfandiari N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res Lett, 16, 1-20 (2021) https://doi.org10.1186/s11671-021-03553-8.
Nisini R, Poerio N, Mariotti S, De Santis F, Fraziano M. The multirole of liposomes in therapy and prevention of infectious diseases. Front Immunol, 9, 1-23 (2018) https://doi.org/10.3389/fimmu.2018.00155.
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther, 3, 1-19 (2018) https://doi.org/10.1038/s41392-017-0004-3.
Sharma A, Sharma US. Liposomes in drug delivery: Progress and limitations. Int J Pharm, 154, 123-140 (1997) https://doi.org/10.1016/S0378-5173(97)00135-X.
Gonda A, Zhao N, Shah J, Calvelli HR, Kantamneni H, Francis N, Ganapathy V. Engineering tumor-targeting nanoparticles as vehicles for precision nanomedicine. Med One, 4, 190021 (2019) https://doi.org/10.20900/mo.20190021.
Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, Deng Y. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci, 10, 81–98 (2015) https://doi.org/10.1016/j.ajps.2014.09.004.
Huang L, Teng W, Cao J, Wang J. Liposomes as Delivery System for Applications in Meat Products. Foods, 11, 1-17 (2022) https://doi.org/10.3390/foods11193017.
Han B, Yang Y, Chen J, Tang H, Sun Y, Zhang Z, Wang Z, Li Y, Li Y, Luan X, Li Q, Ren Z, Zhou X, Cong D, Liu Z, Meng Q, Sun F, Pei J. Preparation, Characterization, and Pharmacokinetic Study of a Novel Long-Acting Targeted Paclitaxel Liposome with Antitumor Activity. Int J Nanomed. 15, 553-571 (2020) https://doi.org/10.2147/IJN.S228715.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics, 9, 1–33 (2017) https://doi.org/10.3390/pharmaceutics9020012.
Nardecchia S, Sanchez-Moreno P, Vicente J, Marchal J, Boulaiz H. Clinical trials of thermosensitive nanomaterials: an overview. Nanomaterials, 9, 1-23 (2019) https://doi.org/10.3390/nano9020191.
He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B, 9, 36-48 (2019) https://doi.org/10.1016/j.apsb.2018.06.005.
Laouini A, Jaafar MC, Limayem BI, Sfar S, Charcosset C, Fessi H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol, 1, 147–168 (2012) http://dx.doi.org/10.1166/jcsb.2012.1020.
Mirzavi F, Barati M, Soleimani A, Vakili GR, Jaafari MR, Soukhtanloo M. A review on liposome-based therapeutic approaches against malignant melanoma. Int J Pharm, 599, 1-11 (2021) https://doi.org/10.1016/j.ijpharm.2021.120413.
Wang G, Li R, Parseh B, Du G. Prospects and challenges of anticancer agents’ delivery via chitosan-based drug carriers to combat breast cancer: A review. Carbohydr Polym, 268, 1-16 (2021) https://doi.org/10.1016/j.carbpol.2021.118192.
Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine, 30, 2256–2272 (2012) https://doi.org/10.1016/j.vaccine.2012.01.070.
Taha EI, El-Anazi MH, El-Bagory IM, Bayomi MA. Design of liposomal colloidal systems for ocular delivery of ciprofloxacin. Saudi Pharm. J, 22, 231–239 (2014) https://doi.org/10.1016/j.jsps.2013.07.003.
Han Y, Gao Z, Chen L, Kang L, Huang W, Jin M, Wang Q, Bae YH. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B, 9, 902–922 (2019) https://doi.org/10.1016/j.apsb.2019.01.004.
Mirtaleb MS, Shahraky MK, Ekrami E, Mirtaleb A. Advances in biological nano-phospholipid vesicles for transdermal delivery: A review on applications. J. Drug Delivery Sci. Technol, 61, 1-11 (2021) https://doi.org/10.1016/j.jddst.2021.102331.
Liu W, Hou Y, Jin Y, Wang Y, Xu X, Han J. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Technol, 104, 177–189 (2020) https://doi.org/10.1016/j.tifs.2020.08.012.
Himeno T, Konno Y, Naito N. Liposomes for Cosmetics. Cosmetic Science and Technology, 31, 539–549 (2017) http://dx.doi.org/10.1016/B978-0-12-802005-0.00031-8.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed, 10, 975-999 (2015) https://doi.org/10.2147/IJN.S68861.
Song H, Hart SL, Du Z. Assembly strategy of liposome and polymer systems for siRNA delivery. Int J Pharm, 592, 1-53 (2021) https://doi.org/10.1016/j.ijpharm.2020.120033.
Kumbham S, Ajjarapu S, Ghosh B, Biswas S. Current trends in the development of liposomes for chemotherapeutic drug delivery. J Drug Del Sci Tech, 87, 104854 (2023) https://doi.org/10.1016/j.jddst.2023.104854.
Gatto MS, Johnson MP, Najahi-Missaoui W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life, 14, 672 (2024) https://doi.org/10.3390/life14060672.
Lan HR, Zhang YN, Han YJ, Yao SY, Yang MX, Xu XG, Mou XZ, Jin KT. Multifunctional nanocarriers for targeted drug delivery and diagnostic applications of lymph nodes metastasis: a review of recent trends and future perspectives. J Nanobiotechnology, 21, 247 (2023). https://doi.org/10.1186/s12951-023-01990-4.
Alexandros GD, Aikaterini S, Anthi P, Ioannis S. Vizirianakis, Dimitrios G. Fatouros, Liposome stability and integrity, Liposomes in Drug Delivery, 5, 89-121 (2024) https://doi.org/10.1016/B978-0-443-15491-1.00022-5.
Han JY, Chen Z, Devoe DL. Scalable Liposome Synthesis by High Aspect Ratio Microfluidic Flow Focusing. Methods Mol Biol., 2622, 87-93 (2023) https://doi.org/10.1007/978-1-0716-2954-3_7.
Yuwei W, David WG. Regulatory considerations specific to liposome drug development as complex drug products. Drug Delivery, 2, 1-8 (2022) http://dx.doi.org/10.3389/fddev.2022.901281.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett, 8, 1-9 (2013) https://doi.org/10.1186/1556-276X-8-102.
Emami S, Azadmard-Damirchi S, Peighambardoust SH, Valizadeh H, Hesari J. Liposomes as carrier vehicles for functional compounds in the food sector. J. Exp. Nanosci, 11, 737–759 (2016) https://doi.org/10.1080/17458080.2016.1148273.
Subczynski WK, Raguz M, Widomska J. Multilamellar Liposomes as a Model for Biological Membranes: Saturation Recovery EPR Spin-Labeling Studies. Membranes, 12, 1-14 (2022) https://doi:10.3390/membranes12070657.
Olusanya TOB, Ahmad RRH, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules, 23, 1–17 (2018) https://doi.org/10.3390/molecules23040907.
Hamid MSS, Hatwar PR, Ravindrakumar LA. comprehensive review on Liposomes: As a novel drug delivery system. GSC Bio Pharm Sci, 27, 199–210 (2024) https://doi.org/10.30574/gscbps.2024.27.1.0121.
Drescher S, van HP. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics, 12, 1-36 (2020) https://doi.org/10.3390/pharmaceutics12121235.
Mozafari MR. Nanomaterials and Nano systems for Biomedical Applications. Springer, 1, 83-98 (2007) https://doi.org/10.1007/978-1-4020-6289-6.
Sackmann E. Membrane bending energy concept of vesicle-and cell-shapes and shape-transitions. FEBS Letters, 346, 3–16 (1994) https://doi.org/10.1016/0014-5793(94)00484-6.
Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon, 8, 1-15 (2022) https://doi:10.1016/j.heliyon.2022.e09394.
Pavlovic N, Mijalkovic J, Balanc B, Lukovic N, Knezevic-Jugovic Z. Role of Cholesterol in Modifying the Physical and Stability Properties of Liposomes and In Vitro Release of VitaminB12. Eng. Proc, 99, 1-9 (2025) https://doi.org/10.3390/engproc2025099010.
Lee E, Kim A, Oh YK, Kim CK. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials. 26, 205–210 (2005) https://doi.org/10.1016/j.biomaterials.2004.02.020.
Tian Y, Chen L, Zhang W. Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants span 80/tween 80. J Dispersion Sci Technol. 37, 1511–1517 (2016) https://doi.org/10.1080/01932691.2015.1048806.
Gangwar M, Singh R, Goel RK, Nath G. Recent advances in various emerging vesicular systems: an overview. Asian Pac J Trop Biomed. 2, 1176–1188 (2012) http://dx.doi.org/10.1016/S2221-1691(12)60381-5.
Pires IS, Suggs JR, Carlo IS, Yun D, Hammond PT, Irvine DJ. Surfactant-Mediated Assembly of Precision-Size Liposomes. Chem Mater, 36, 7263–7273 (2024) https://doi.org/10.1021/acs.chemmater.4c01127.
Chen R, Li R, Liu Q, Bai C, Qin B, Ma Y, Han J. Ultradeformable liposomes: a novel vesicular carrier for enhanced transdermal delivery of procyanidins: effect of surfactants on the formation, stability, and transdermal delivery. AAPS PharmSciTech, 18, 1823–1832 (2017) https://doi.org/10.1208/s12249-016-0661-5.
Souto EB, Macedo AS, Dias-Ferreira J, Cano A, Zielińska A, Matos CM. Elastic and ultradeformable liposomes for transdermal delivery of active pharmaceutical ingredients (APIs). Int J Mol Sci, 22, 9743 (2021) https://doi.org/10.3390/ijms22189743.
Gao L, Zhang L, He F, Chen J, Zhao M, Li S, Wu H, Liu Y, Zhang Y, Ping Q, Hu L, Qiao H. Surfactant Assisted Rapid-Release Liposomal Strategies Enhance the Antitumor Efficiency of Bufalin Derivative and Reduce Cardiotoxicity. Int J Nanomedicine, 16, 3581-3598 (2021) https://doi.org/10.2147/IJN.S313153.
Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A. (2004). Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). Journal of Controlled Release, 98, 195–207 (2004) https://doi.org/10.1016/j.jconrel.2004.04.018.
Okyere D, Manso RH, Tong X, Chen J. Stability of Polyethylene Glycol-Coated Copper Nanoparticles and Their Optical Properties. Coatings, 12, 1-13 (2022) https://doi.org/10.3390/coatings12060776.
Lamichhane N, Udayakumar TS, DSouza WD, Simone CB, Raghavan SR, Polf J, Mahmood J. Liposomes: Clinical Applications and Potential for image-Guided Drug Delivery. Molecules, 23, 1-17 (2018) https://doi.org/10.3390/molecules23020288.
Cagdas M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery, application of nanotechnology in drug delivery. IntechOpen, 1, 1-50 (2014) http://dx.doi.org/10.5772/58459.
Taira MC, Chairamoni NS, Pecuch KM, Alonso-Romanowski S. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Delivery, 11, 123–128 (2004) https://doi.org/10.1080/10717540490280769.
Sagahara S, Kajiki M, Kuriyama H, Kobayashi T. Complete regression of Xenografted human carcinomas by a paclitaxel-carboxymethyl dextran conjugate (AZ10992). Journal Controlled Release, 117, 40–50 (2006) https://doi.org/10.1016/j.jconrel.2006.10.009.
Pasarin D, Ghizdareanu AI, Enascuta CE, Matei CB, Bilbie C, Paraschiv-Palada L, Veres PA. Coating Materials to Increase the Stability of Liposomes. Polymers, 15, 1-30 (2023) https://doi.org/10.3390/polym15030782.
Andra VVSN, Pammi SVN, Bhatraju LVKP, Ruddaraju LK. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoSci. 12, 274–291 (2022). https://doi.org/10.1007/s12668-022-00953-7.
Zhang, H. Thin film hydration followed by Extrusion Method for Liposome Preparation. Methods Mol Biol, 1522, 17–22 (2017) https://doi.org/10.1007/978-1-4939-6591-5_2.
Bnyan R, Cesarini L, Khan I, Roberts M, Ehtezazi T. The effect of ethanol evaporation on the properties of inkjet produced liposomes. DARU J Pharm Sci, 28, 2020 https://doi.org/10.1007/s40199-020-00340-1.
Handa T, Naito S, Hiramatsu M, Tsuboi M. (2006). Thermal SiO and H13CO+ line observations of the dense molecular cloud G0.11–0.11 in the Galactic Center Region. Astrophys J, 636, 261–266 (2006) https://doi.org/10.1086/497881.
Shaheen SM, Ahmed FRS, Hossen MN, Ahmed M, Amran MS, UI-Islam MA. Liposomes as a carrier for the advanced drug delivery. Pak J Biol Sci, 9, 1181–1191. (2006) http://dx.doi.org/10.3923/pjbs.2006.1181.1191.
Nkanga CI, Krause RWM. Encapsulation of Isoniazid-conjugated Phthalocyanine-In-Cyclodextrin-In-Liposomes Using Heating Method. Scientific Reports, 9, 1-16 (2019) https://doi.org/10.1038/s41598-019-47991-y.
Jahadi M, Darani KK, Ehsani MR, Mozafari MR, Saboury AA, Pourhosseini PS. (2015). The Encapsulation of Flavourzyme in Nano liposome by heating method. J Food Sci Tech, 52, 2063–2072 (2015) https://doi.org/10.1007/s13197-013-1243-0.
Rane BR, Jain AS, Mane NP, Patil V, Patil MS, Bavaskar KR. Fabrication and evaluation of carbocisteine-loaded solid lipid nanoparticles to treat pulmonary infections. Journal of Applied Pharmaceutical Research, 12, 122–36 (2024) https://doi.org/10.69857/joapr.v12i6.661.
Jahn A, Vreeland WN, Devoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir, 23, 6289–6293 (2007) https://doi.org/10.1021/la070051a.
Yu B, Lee JR, Lee LJ. Microfluidic Methods for Production of Liposomes. Methods in Enzymology, 465, 129–141 (2009) https://doi.org/10.1016/S0076-6879(09)65007-2.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour SY, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: Classification, preparation, and applications. Nanos Res Lett, 8, 1-9, (2013) https://doi.org/10.1186/1556-276x-8-102.
Pauli G, Tang WL, Li SD. (2019). Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics, 11, 1-12 (2019) https://doi.org/10.3390/pharmaceutics11090465.
Nambiar NR, Gaur S, Ramachandran G, Pandey RS, Sabitha M, Nath LR, Dutta T, Sudheesh MS. Remote loading in liposome: a review of current strategies and recent developments. J Liposome Res, 34, 658-670 (2024) https://doi.org/10.1080/08982104.2024.2315449.
Yen TT. H Dan, LN Hoang, LD Tung, BT, Minh Hue PT. Preparation and characterization of freeze dried liposomes loaded with Amphotericin B. Current Drug Therapy, 14, 65-73 (2019) https://doi.org/10.2174/1574885514666181217130259.
Hirsch M, Ziroli V, Helm M, Massing U. Preparation of small amounts of sterile siRNA – liposomes with high entrapping efficiency by dual asymmetric centrifugation (DAC). J Cont Rel, 135, 80–88 (2009) https://doi.org/10.1016/j.jconrel.2008.11.029.
Maja L, Zeljko K, Mateja P. Sustainable technologies for liposome preparation. J Superc Flui, 165, 1049–1084 (2020) https://doi.org/10.1016/j.supflu.2020.104984.
Trucillo P, Campardelli R, Scognamiglio M, Reverchon E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J CO2 Utili, 32, 119–127 (2019) https://doi.org/10.1016/j.jcou.2019.04.014.
Chakravarty P, Famili A, Nagapudi K, Al-Sayah MA. Using supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics, 11, 1-32 (2019) https://doi.org/10.3390/pharmaceutics11120629.
Pasquali I, Bettini R. Are pharmaceutics really going supercritical. Int J Pharm, 364, 176–187 (2008) https://doi.org/10.1016/j.ijpharm.2008.05.014.
Pawar N, Agrawal S, Methekar R. Continuous Antisolvent Crystallization of α-Lactose Monohydrate: Impact of Process Parameters, Kinetic Estimation, and Dynamic Analysis. Org Pro Res Dev, 23, 2394–2404 (2019) https://doi.org/10.1021/acs.oprd.9b00301.
Huang Z, Li X, Zhang T, Song Y, She Z, Li J, Deng Y. Progress involving new techniques for liposome preparation. Asian J Pharm Sci, 9, 176–182 (2014) https://doi.org/10.1016/j.ajps.2014.06.001.
Bagheri H, Mansoori GA, Hashemipour H. A novel approach to predict drugs solubility in supercritical solvents for RESS process using various cubic EoS-mixing rule. J Mol Liq, 261, 174–188 (2018) https://doi.org/10.1016/j.molliq.2018.03.081.
Zhao L, Temelli F. Preparation of anthocyanin-loaded liposomes using an improved supercritical carbon dioxide method. Inno Food Sci Emer Tech, 39, 119–128 (2017) https://doi.org/10.1016/j.ifset.2016.11.013.
Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: Advancements and innovation in the manufacturing process. Adv Drug Deliv Rev, 154-155, 102–122 (2020) https://doi:10.1016/j.addr.2020.06.016.
Chaves MA, Baldino L, Pinho SC, Reverchon E. Supercritical CO2 assisted process for the production of mixed phospholipid nanoliposomes: Unloaded and vitamin D3-loaded vesicles. J Food eng, 316, 110851 (2022) https://doi.org/10.1016/j.jfoodeng.2021.110851.
Remo E, Paola L. Liposomes: Bridging the gap from lab to pharmaceuticals. Current Opinion in Colloid & Interface Science, 75, 101875 (2025) https://doi.org/10.1016/j.cocis.2024.101875.
Lara LA, Avila EM, Gutierrez MAL, Alvarez EO, Olive KI. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem Phys Lip, 230, 1-50 (2020) https://doi.org/10.1016/j.chemphyslip.2020.104934.
Pitkanen L. Potential of size-exclusion chromatography and asymmetric flow field-flow fractionation in separation and characterization of plant polysaccharides. J Chromat A, 1748, 465862 (2025) https://doi.org/10.1016/j.chroma.2025.465862.
Bokrova J, Marova I, Matouskova P, Pavelkova R. Fabrication of novel PHB-liposome nanoparticles and study on their toxicity in vitro. J Nanop Res, 21, 1-12 (2019) https://doi.org/10.1007/s11051-019-4484-7.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: A case study of cationic, anionic and neutral liposomes. Analyt bioanalyt chem, 409, 5779–5787 (2017) https://doi.org/10.1007/s00216-017-0527-z.
Ong SGM, Ming LC, Lee KS. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics, 8, 1-17, (2016) https://doi.org/10.3390/pharmaceutics8030025.
Shamshiri MK, Jaafari MR, Badiee A. Preparation of liposomes containing IFN-gamma and their potentials in cancer immunotherapy: In vitro and in vivo studies in a colon cancer mouse model. Life Sciences, 264, 11860–11865 (2021) https://doi.org/10.1016/j.lfs.2020.118605.
Liu X, Zhang L, Jiang W, Yang Z, Gan Z, Yu C, Chen H. In vitro and in vivo evaluation of liposomes modified with polypeptides and red cell membrane as a novel drug delivery system for myocardium targeting. Drug Delivery, 27, 599–606 (2020) https://doi.org/10.1080/10717544.2020.1754525.
Amiri M, Gholami T, Amiri O, Pardakhti A, Ahmadi M, Akbari A, Amanatfard A, Niasari MS. The magnetic inorganic-organic nanocomposite based on ZnFe2O4 Imatinib-liposome for biomedical applications, In vivo and In vitro study. J Alloys Comp, 849, 15660–15664 (2020) https://doi.org/10.1016/j.jallcom.2020.156604.
Wang C, Gamage PL, Jiang W, Jiang W, Mudalige TK. Excipient-related impurities in liposome drug products. Int J Pharm, 657, 124164 (2024) https://doi.org/10.1016/j.ijpharm.2024.124164.
Seyedeh AM, Vanessa B, Matteo P, Amirhossein S, Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Seminars in Cancer Biology, 69, 337-348 (2021) https://doi.org/10.1016/j.semcancer.2019.09.025.
Published
How to Cite
Issue
Section
Copyright (c) 2026 Debatri Roy, Beduin Mahanti, Sudipta Das, Arnab Samanta, Setu Majumder

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















