Design and evaluation of amphotericin b and luliconazole nanoemulsions for targeted antifungal delivery
DOI:
https://doi.org/10.69857/joapr.v14i1.1229Keywords:
Nanoemulsion, fungal infection, Amphotericin B, Luliconazole, AqbD, Particle size, zeta potentialAbstract
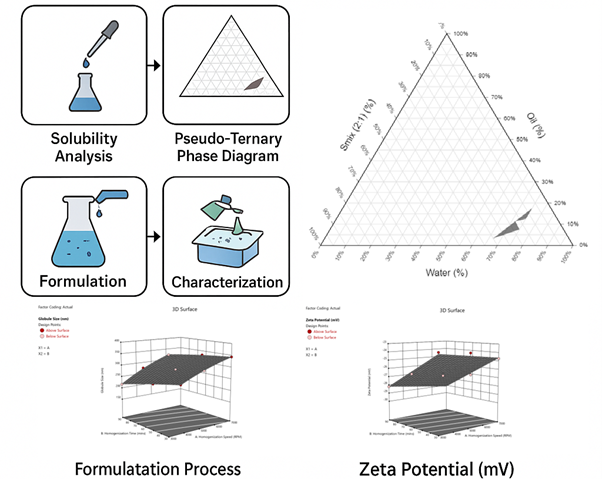
Background: Drugs like Amphotericin B and Luliconazole, which are poorly soluble in water and undergo significant first-pass metabolism, often show low bioavailability. Using nanoemulsion-based delivery systems can enhance absorption and efficacy in the treatment of fungal infections. This study aimed to develop and optimize nanoemulsion formulations of Amphotericin B and Luliconazole to improve their solubility and stability and to demonstrate potential for enhanced bioavailability. Methods: Preliminary characterization of Amphotericin B and Luliconazole included solubility analysis in various solvents, melting point determination, particle size, zeta potential, FTIR spectroscopy, DSC, and XRD. Amphotericin B was further evaluated using a validated RP-HPLC method and subjected to forced degradation studies. Pseudo-ternary phase diagrams were constructed to identify suitable Smix ratios for nanoemulsion formation. Formulations were prepared by homogenization and optimized using a central composite design. Key variables included globule size, zeta potential, homogenization speed, and time. Results and Discussion: The optimized Amphotericin B nanoemulsion (NE-02-8) exhibited a globule size of 168.2 nm, zeta potential of –28.9 mV, PDI of 0.578, drug content of 99.28%, and 99.48% transmittance. Statistical optimization using a Central Composite Design (CCD) confirmed that homogenization speed and time significantly influenced globule size (p < 0.05) and zeta potential (p < 0.05). In contrast, the Luliconazole nanoemulsion showed a globule size of 327.5 nm and a zeta potential of –27.9 mV. Conclusion: Nanoemulsion formulations of Amphotericin B and Luliconazole demonstrated enhanced solubility, stability, and physicochemical properties, indicating their potential to improve drug solubilization and stability relative to conventional formulations.
Downloads
References
Choudhury H, Gorain B, Pandey M, et al. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci., 106(7), 1736–51 (2017) https://doi.org/10.1016/j.xphs.2017.03.042.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm., 366(1), 170–84 (2009) https://doi.org/10.1016/j.ijpharm.2008.10.003.
Yang J, Liang Z, Lu P, et al. Development of a luliconazole nanoemulsion as a prospective ophthalmic delivery system for the treatment of fungal keratitis: in vitro and in vivo evaluation. Pharmaceutics, 14(10), 2052 (2022) https://doi.org/10.3390/pharmaceutics14102052.
Yusof N, David SR, Mumin NH, Ahmad L, Rajabalaya R. Formulation and development of Commiphora myrrha based polyherbal nanoemulsion mouthwash and assessment of its anti-oxidant and cytotoxicity activity. Journal of Applied Pharmaceutical Research, 12, 87–101 (2024) https://doi.org/10.69857/joapr.v12i5.679.
dos Santos Matos AP, Lopes DC, Peixoto ML, et al. Development, characterization, and anti-leishmanial activity of topical amphotericin B nanoemulsions. Drug Deliv. Transl. Res., 10(6), 1552–70 (2020) https://doi.org/10.1007/s13346-020-00821-5.
Kumari S, Alsaidan OA, Mohanty D, et al. Development of soft luliconazole invasomes gel for effective transdermal delivery: optimization to in-vivo antifungal activity. Gels, 9(8), 626 (2023) https://doi.org/10.3390/gels9080626.
Padaraju A, Dwivedi F, Kumar G. Microemulsions, nanoemulsions and emulgels as carriers for antifungal antibiotics. Ther. Deliv., 14(11), 721–40 (2023) https://doi.org/10.4155/tde-2023-0076.
Sharma AD, Kaur I, Chauhan A. Anti-aspergillosis and anti-mucormycosis potential of eucalyptus essential oil-based O/W nanoemulsions containing azole-based drugs from Eucalyptus globulus. J. Umm Al-Qura Univ. Appl. Sci., 10(2), 313–29 (2024) https://doi.org/10.1007/s43994-023-00108-8.
Aderibigbe BA. Nanotherapeutics for the delivery of antifungal drugs. Ther. Deliv., 15(1), 55–76 (2024) https://doi.org/10.4155/tde-2023-0090.
Nagaraj S, Manivannan S, Narayan S. Potent antifungal agents and use of nanocarriers to improve delivery to the infected site: A systematic review. J. Basic Microbiol., 61(10), 849–73 (2021) https://doi.org/10.1002/jobm.202100204.
Nosratabadi M, Akhtari J, Jaafari MR, et al. In vitro activity of nanoliposomal amphotericin B against terbinafine-resistant Trichophyton indotineae isolates. Int. Microbiol., 1(1), 1–7 (2024) https://doi.org/10.1007/s10123-024-00617-4.
Mahaling B, Baruah N, Dinabandhu A. Drug delivery systems for infectious eye diseases: advancements and prospects. J. Nanotheranostics, 5(4), 133–66 (2024) https://doi.org/10.3390/jnt5040010.
Abdalla A, El-Sawy HS, Ramadan AA. Emergence of advanced antifungal-delivery approaches for the treatment of tinea pedis. ERU Res. J., 3(2), 1058–89 (2024) https://doi.org/10.21608/erurj.2024.227252.1064.
Asaf MB, Khairiyah, Kurniawan I, et al. Amphotericin B nanocrystals integrated with bilayer dissolving microneedles: a new strategy for transmucosal delivery of amphotericin B to improve the effectiveness of oral candidiasis therapy. J. Pharm. Investig., 1(1), 1–9 (2025) https://doi.org/10.1007/s40005-025-00726-w.
Arimoto S, Inagaki K, Todokoro D, et al. Antifungal efficacy of luliconazole in an experimental rabbit model of fungal keratitis caused by Fusarium solani. Mycopathologia, 188(5), 775–82 (2023) https://doi.org/10.1007/s11046-023-00783-5.
Ahuja A, Bajpai M. Nanoformulations insights: a novel paradigm for antifungal therapies and future perspectives. Curr. Drug Deliv., 21(9), 1241–72 (2024) https://doi.org/10.2174/0115672018270783231002115728.
Nagpal M, Kaur M. Nanomaterials for skin antifungal therapy: an updated review. J. Appl. Pharm. Sci., 11(1), 015–25 (2021) https://doi.org/10.7324/JAPS.2021.11s102.
Nogueira NC, de Sá LL, de Carvalho AL. Nanostructured lipid carriers as a novel strategy for topical antifungal therapy. AAPS PharmSciTech, 23(1), 32 (2021) https://doi.org/10.1208/s12249-021-02181-w.
Raina N, Rani R, Thakur VK, Gupta M. New insights in topical drug delivery for skin disorders: from a nanotechnological perspective. ACS Omega, 8(22), 19145–67 (2023) https://doi.org/10.1021/acsomega.2c08016.
Published
How to Cite
Issue
Section
Copyright (c) 2026 Pravin N. Kirdat, Meenakshi B. Patel

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















